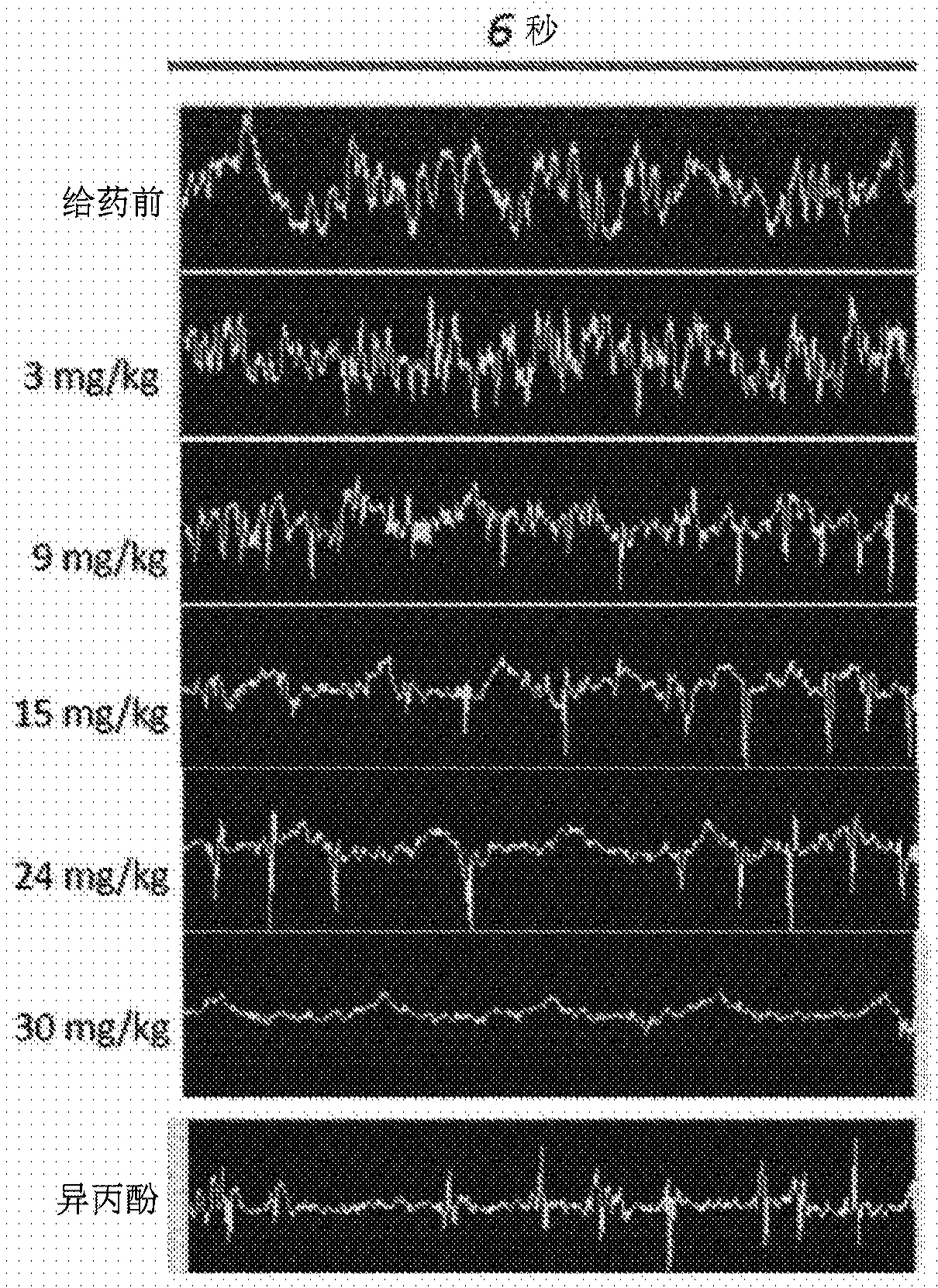
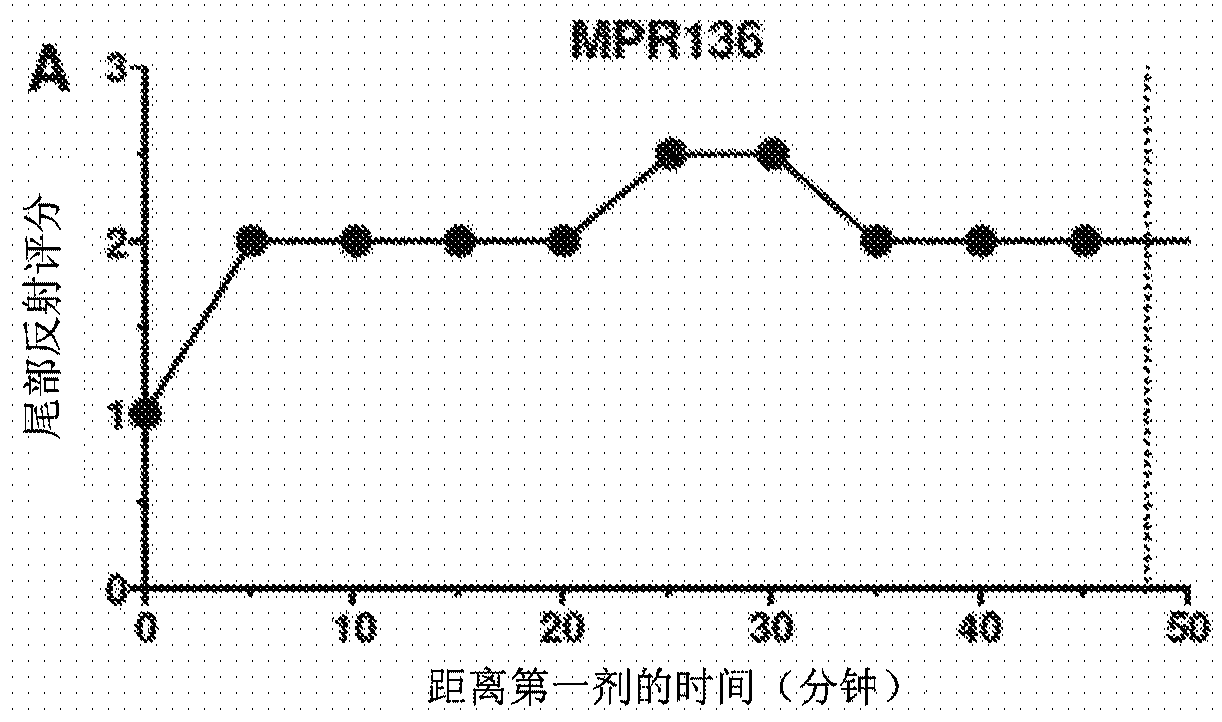
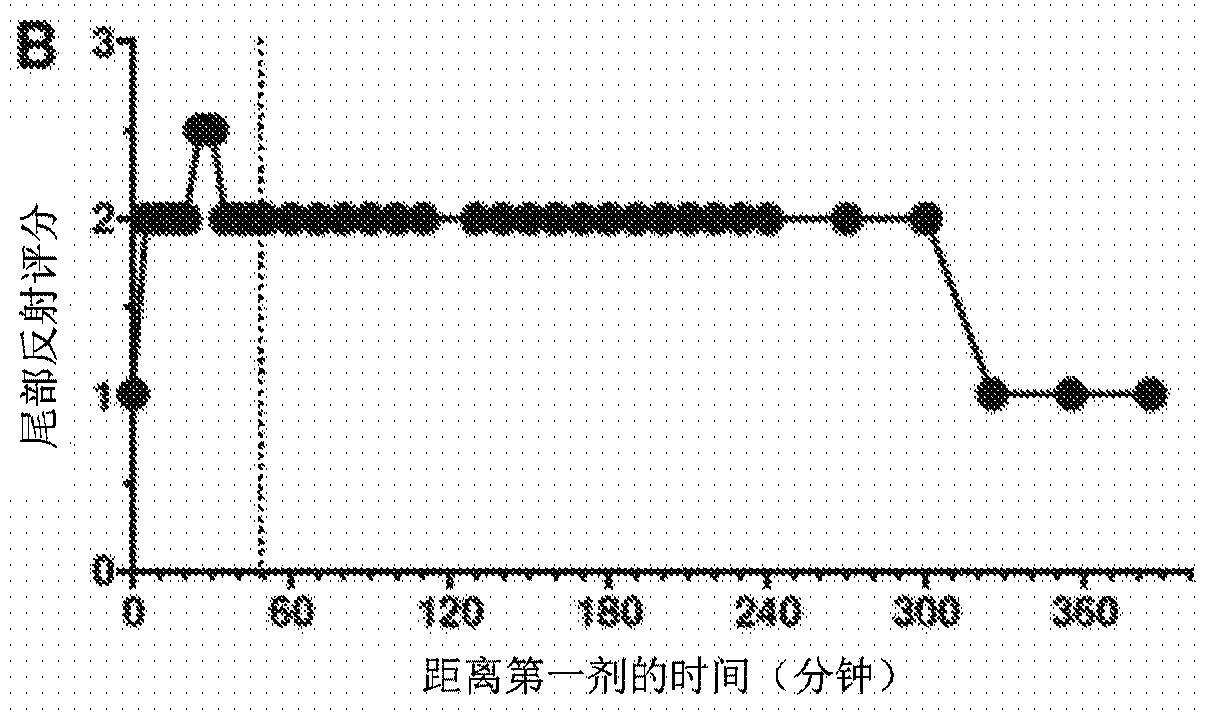
Method of administering a neurosteroid to effect electroencephalographic (EEG) burst suppression
A technology of outbreak suppression and steroids, applied in nervous system diseases, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0131] Example 1. Preparation of Injectable Ganaxolone CAPTISOL Formulation
[0132] The following formulations were used as positive controls. For the preparation of injectable solutions, excess ganaxolone was added to 400 mg / mL CAPTISOL in water. The solution was shaken at least overnight and filtered through a 0.45 micron filter. The ganaxolone concentration of the filtered solution was determined by HPLC. Ganaxolone / CAPTISOL solution (7.68 mg / mL ganaxolone in 400 mg / mL CAPTISOL aqueous solution) was diluted in saline to obtain 3.84 mg / mL, 0.77 mg / mL and 0.39 mg / mL ganaxol in 0.9% saline Solone solution. All solutions were clear without any visible precipitate. After freezing and thawing, the ganaxolone solution remained free of any visible precipitate.
Embodiment 2
[0133] Example 2. Injectable Ganaxolone-CAPTISOL Solution (5MG / ML)
[0134] This formulation was also used as a positive control. Ganaxolone (0.50 g) was manually mixed with a small amount (approximately 20 mL) of 30% w / v CAPTISOL solution in sterile water for injection with a spatula to form a homogeneous paste. An additional amount (about 40 mL) of 30% w / v CAPTISOL solution was then added to obtain a slurry. The suspension was stirred for 20 minutes using a magnetic stir bar. Sonication was performed for 2 hours (h) using a probe sonicator. While sonicating, additional 30% w / v CAPTISOL solution was added until the total amount of CAPTISOL solution reached 99.58 mL. The stirred formulation was then heated at 68.5°C for 2.5 hours to obtain a solution. The heat was removed and the solution was stirred at room temperature for about 2 hours. Volume lost due to evaporation is made up with water. The clear solution was sterile filtered through a 0.2 μm nylon membrane filter. ...
Embodiment 3
[0135] Example 3. Preparation of Ganaxolone Nanosuspension (10% WT) by Wet Bead Milling
[0136] Grinding media with 0.3 mm YTZ beads (yttrium stabilized, Tosoh Corporation, Japan, ZrO 2 +HfO 2 (95wt% (weight %)), Y 2 o 2 (5wt%) Netzsch Mill (Minicer) milled an aqueous solution containing ganaxolone (25g), hydroxyethyl starch (7.5g), sodium deoxycholate (0.5g) and 30% simethicone (1 drop) Slurry (250 g). Two additional portions of solid sodium deoxycholate (0.5 g each) were added at 100 and 130 minutes after the start of milling. The particle size of the abrasive slurry was measured using a Horiba LA-910 laser diffraction particle size analyzer. After milling for 170 minutes, the D50 was 192 nm (188 nm after 1 minute of sonication). At this point, milling was stopped and the milled slurry was kept at room temperature overnight. The next morning, milling was resumed until the total milling time reached 320 minutes, at which point the D50 was 167 nm (169 nm after 1 minute...
PUM
| Property | Measurement | Unit |
|---|---|---|
| D50 | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com