ABPP-based antitumor drug ginsenoside Rg3 active molecular probe, synthesis and applications thereof
A technology of ginsenosides and active molecules, which is applied in the field of chemical biology and can solve problems such as research work that has not been reported yet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
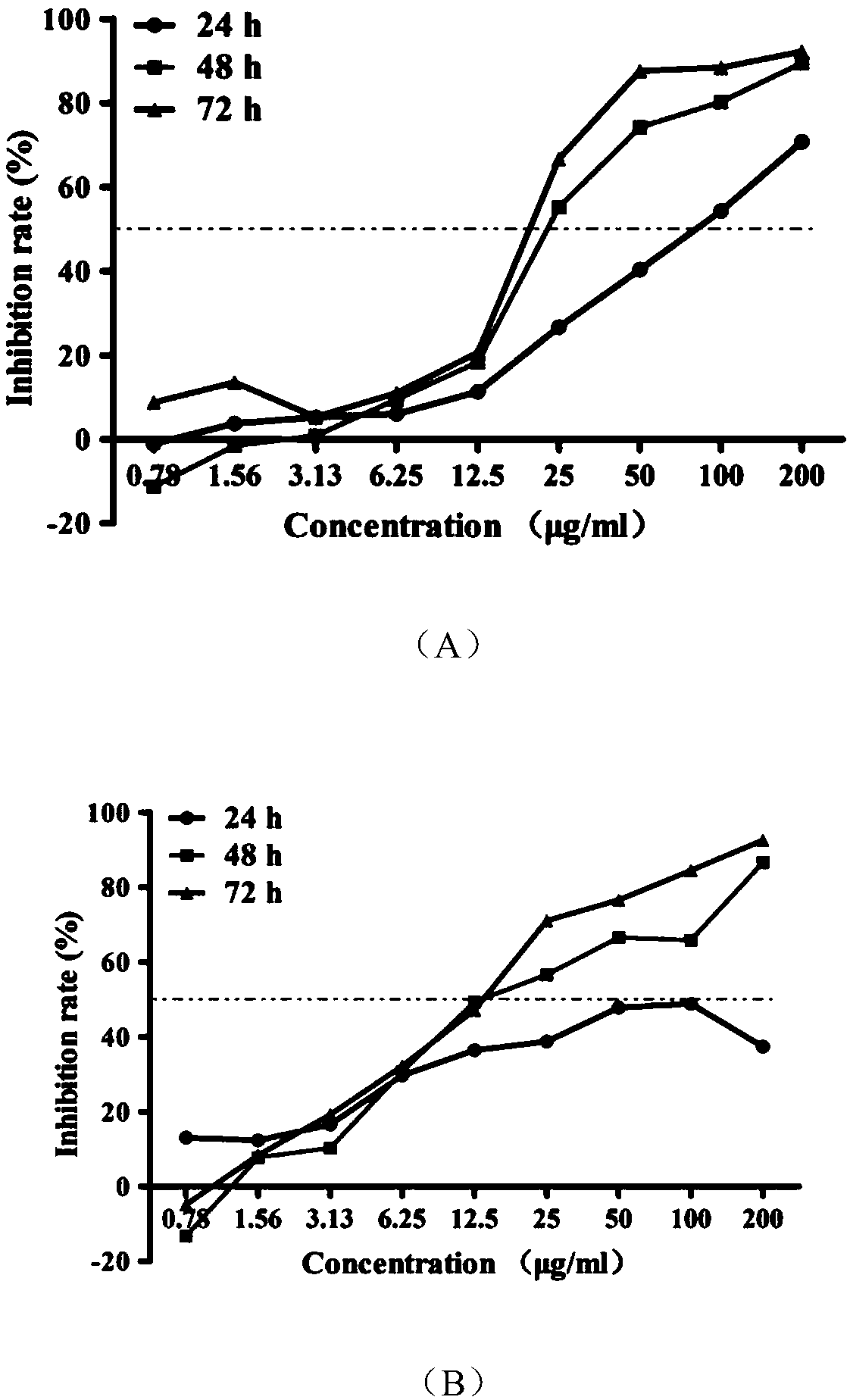
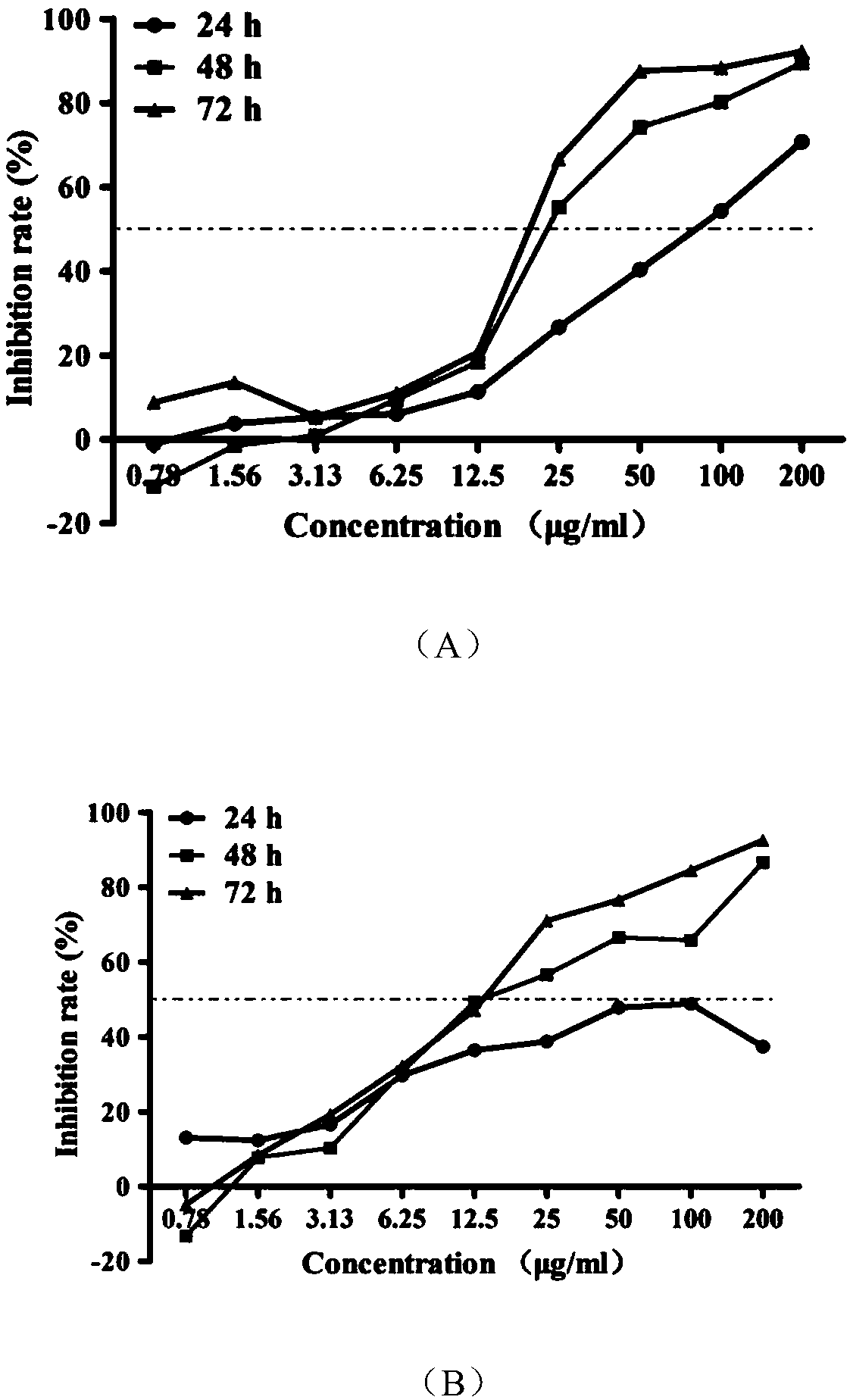
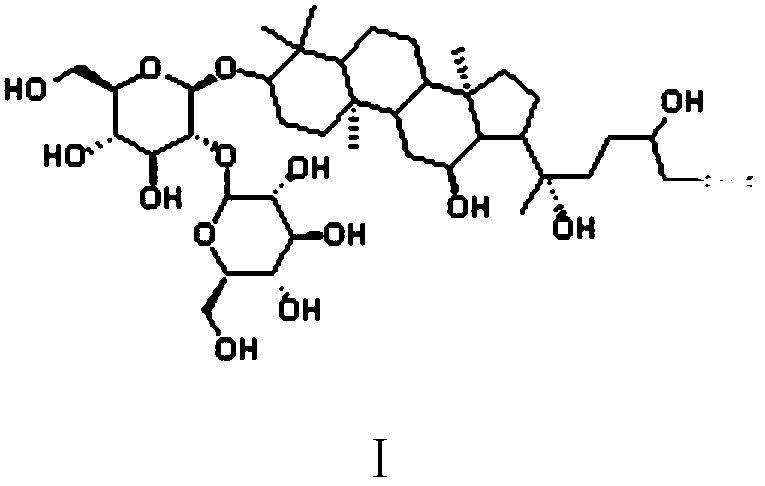
[0020] The structure of the active molecular probe of ginsenoside Rg3 based on ABPP is:
[0021]
[0022] Wherein, the group connected to the rightmost end is an alkynyl group, which is used as a reporter group in the active molecular probe. The specific synthetic route is
[0023]
[0024] The reaction conditions are:
[0025] 20(R)-ginsenoside Rg3 (1g) was dissolved in 100ml of anhydrous pyridine, stirred to obtain 20(R)-ginsenoside Rg3 solution, and the acylating reagent acetyl chloride (2ml) was added dropwise for esterification reaction. The temperature was 80°C, the reaction time was 2h, then anhydrous methanol (5ml) was added to quench the esterification reaction, the pH value of the mixed solution was adjusted to 7.2, filtered, recrystallized; then dissolved in 100ml dichloromethane and acetic acid solution (two The volume ratio of the two reagents is 1:1), and O is introduced at -78°C 3 , until the reaction turns blue, then pass through O 2 remove excess O ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com