Application of Geniposide in the Preparation of Drugs for Treating Multiple Sclerosis
A technology of multiple sclerosis and geniposide, which is applied in the application field of geniposide in the preparation of drugs for the treatment of multiple sclerosis, can solve the problems of unclear mechanism of action and limitation of clinical application, and achieve universality, The effect of obvious curative effect and few side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
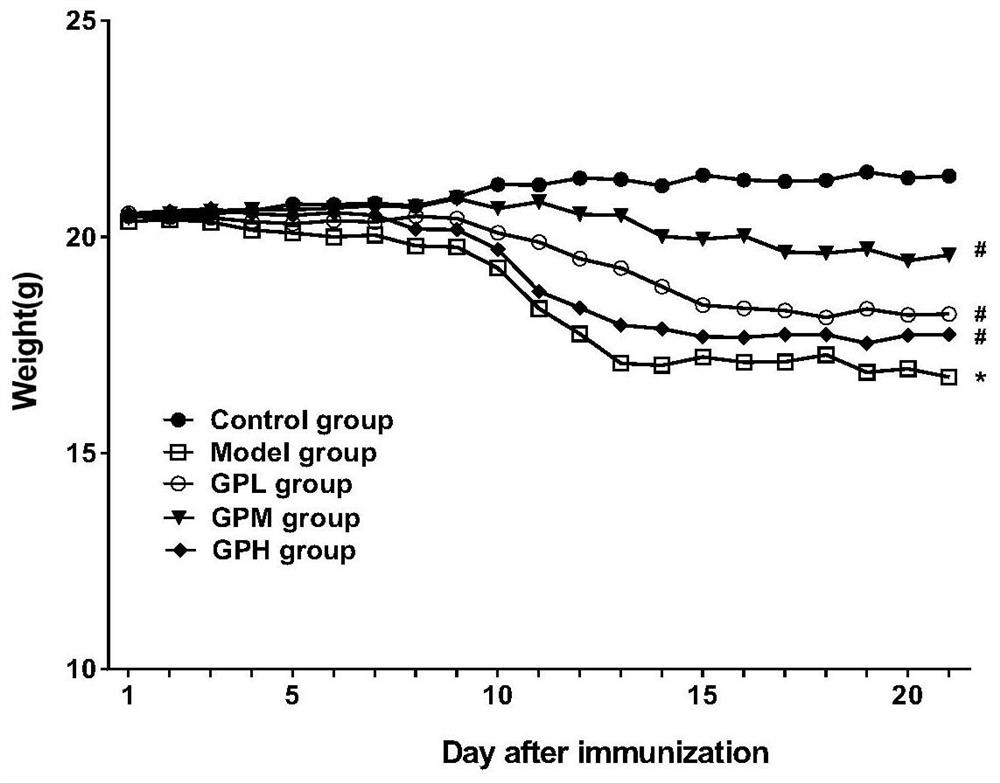
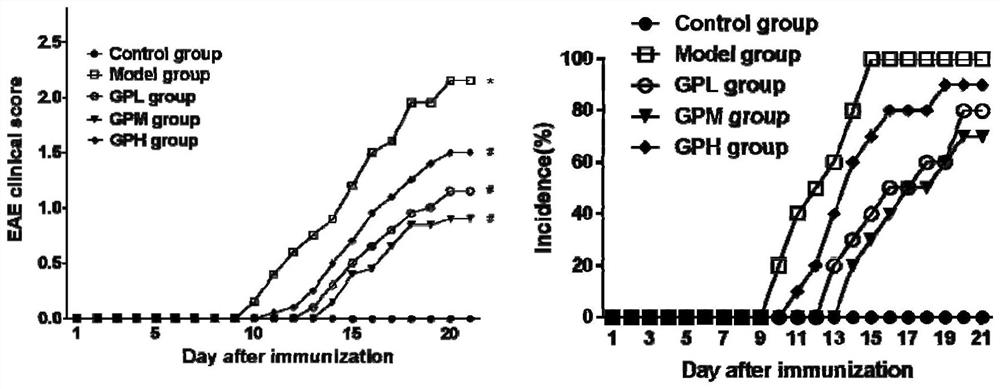
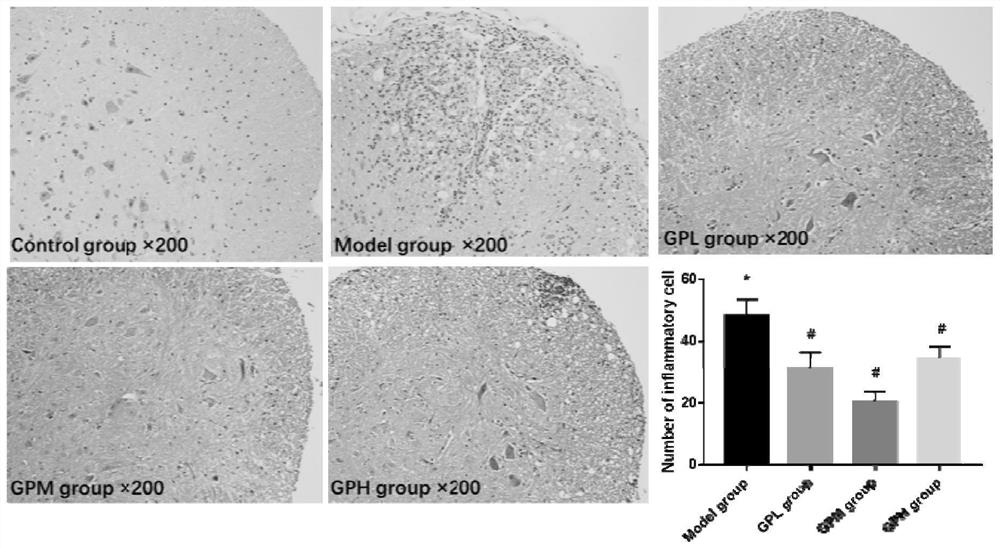
[0037] 1 Experimental animals and reagents
[0038] Experimental animals: C57BL / 6 mice, female, 8 weeks old, weighing 18-22 g, provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (23±1)°C, 12h alternating light and dark lighting, fed sterile common rat food, free access to water.
[0039] Experimental reagents: MOG 35-55 polypeptide, purchased from Mimotopes Peptides Company. Freund's complete adjuvant was purchased from Chondrex; pertussis toxin was purchased from List Biological Laboratories; geniposide was purchased from Jiangxi Linchuan Zhixin Biotechnology Company; ELISA kit was purchased from Wuhan Yunclone Technology Co., Ltd.; flow staining antibody and Pharmingen fixation / permeabilization kit were purchased from BD Company; X1R high-performance general-purpose desktop centrifuge was purchased from ThermoScientific Company; FACS Aria II flow cytometer was purchased from BD Company of the United States.
[0040] 2 Experimental methods
[0041] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com