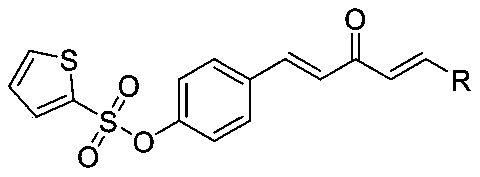
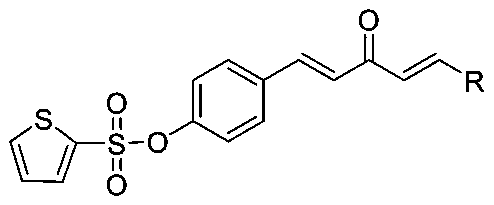
1,4-Pentadiene-3-ketone derivative with thiophene sulphonate and preparation method and application of derivative
A technology of thiophene sulfonate and pentadiene, which is applied in the direction of botany equipment and methods, chemicals for biological control, applications, etc., can solve problems that have not been seen, and achieve good inhibitory activity, high yield, Post-processing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
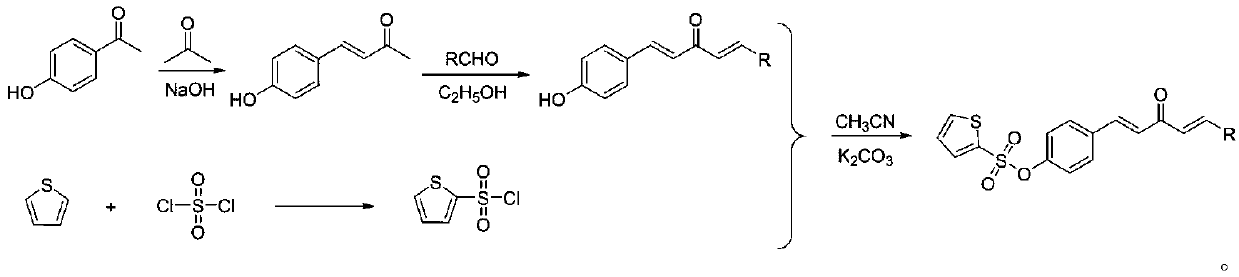
[0025] Synthesis of (4-((1E,4E)-3-oxo-5-phenylpenta-1,4-dien-1-yl)phenylthiophene-2-sulfonate (compound number is A1), Include the following steps:
[0026] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: 4-hydroxybenzaldehyde (5.0g) was added to 50mL of acetone, after stirring for about 20min, the reaction system was ice-bathed for about After 30 min, about 80 mL of 5% NaOH solution was added to the system. After the dropwise addition was completed, the ice bath was removed, and stirred at room temperature for about 24 h. After the reaction is over, transfer the system to a 500mL beaker and add an appropriate amount of ice water, and then use 5% dilute hydrochloric acid solution to adjust the pH of the system to about 5-6. After a large amount of yellow solids precipitate, extract the solids, and finally use ethanol to / water system recrystallized to obtain a yellow solid with a yield of 70%.
[0027] (2) Synthesis of 1-(4-phenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: ...
Embodiment 2
[0030] Synthesis of (4-((1E,4E)-5-(4-chlorophenyl)-3-oxopent-1,4-dien-1-yl)phenylthiophene-2-sulfonate (compound numbered A2), including the following steps:
[0031] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: as in step (1) of Example 1.
[0032] (2) Synthesis of 1-(4-(4-chlorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: as in step (2) of Example 1, The difference is that 4-chlorobenzaldehyde is used as raw material.
[0033] (3) (4-((1E,4E)-5-(4-chlorophenyl)-3-oxopent-1,4-dien-1-yl)phenylthiophene-2-sulfonate Synthesis: as in step (3) of Example 1.
Embodiment 3
[0035] Synthesis of (4-((1E,4E)-5-(2-chlorophenyl)-3-oxopent-1,4-dien-1-yl)phenylthiophene-2-sulfonate (compound numbered A3), including the following steps:
[0036] (1) Synthesis of 4-(hydroxyphenyl)-3-buten-2-one: as in step (1) of Example 1.
[0037] (2) Synthesis of 1-(4-(2-chlorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one: as in step (2) of Example 1, The difference is that 2-chlorobenzaldehyde is used as raw material.
[0038] (3) (4-((1E,4E)-5-(2-chlorophenyl)-3-oxopent-1,4-dien-1-yl)phenylthiophene-2-sulfonate Synthesis: as in step (3) of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com