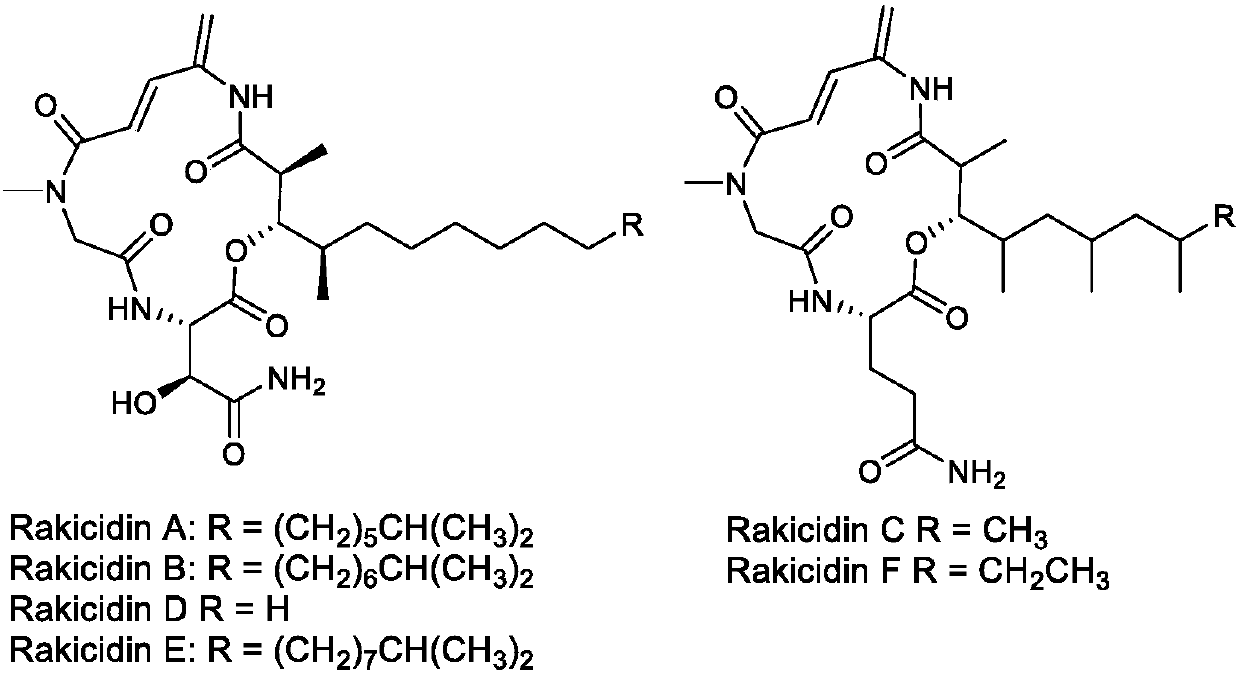
Rakicidin derivatives, and preparation method and application thereof in preparation of anticancer drugs
A technology for derivatives and cancer, which is applied in the field of Rakicidin derivatives, preparation of Rakicidin derivatives, and preparation of drugs for treating cancer. It can solve the problems of low total yield, poor stability, and long total synthesis route, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1: Synthesis of Rakicidin derivatives and salts thereof
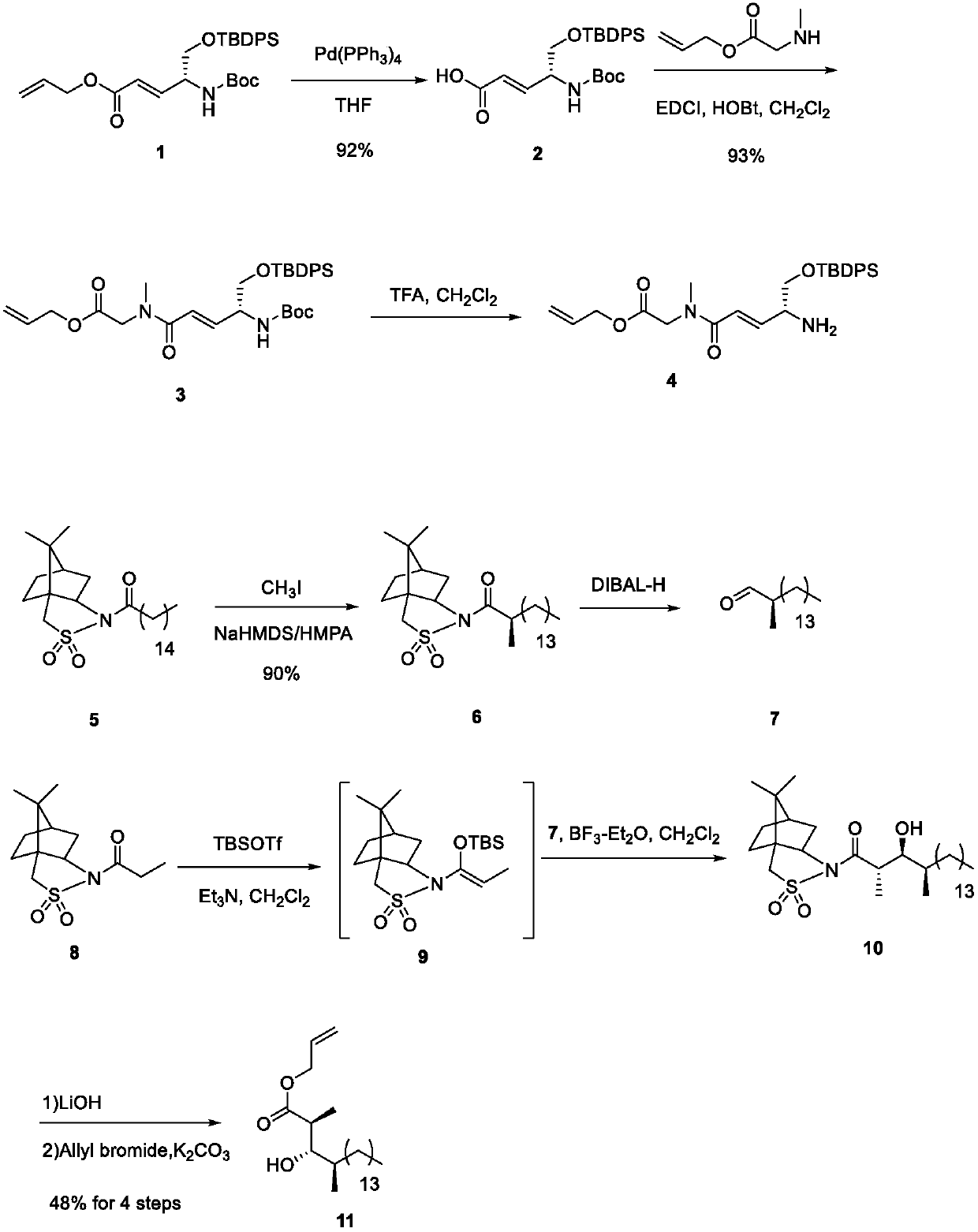
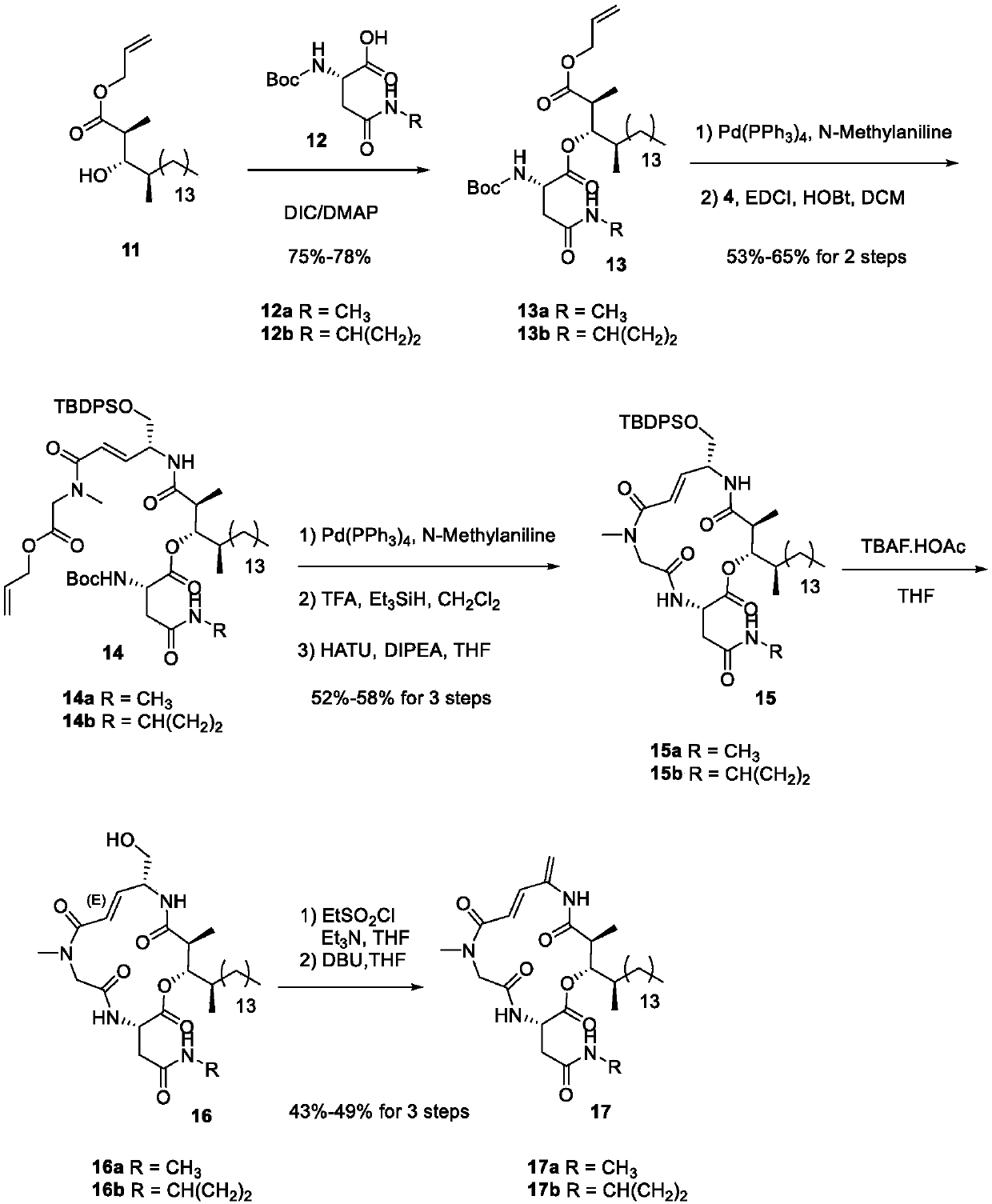
[0016] Please refer to the appendix of the manual for the specific synthetic route figure 2 with instructions attached image 3 ,Specific steps are as follows:
[0017] Under argon protection, tetrahydrofuran (350mL) solution of compound 8 (35.7g, 70.0mmol) was added successively with tetrakistriphenylphosphopalladium (16.19g, 14mmol) and N-methylaniline (15g, 140mmol), at room temperature The reaction was stirred for 1.5 h, and the reaction solution was diluted with ethyl acetate (500 mL). The organic phase was washed twice with 1% HCl (120 mL). The obtained organic phase was dried with anhydrous sodium sulfate, filtered and concentrated, and the obtained crude product was purified by silica gel column to obtain colorless oily compound 2 (30.25g, 92%)
[0018] In a 250mL round bottom flask, compound 2 (19.2g, 40.88mmol), allyl sarcosinate (7.92g, 61.32mmol) and 160mL of dichloromethane obtained i...
Embodiment 2
[0042] Example 2: Normoxic and Hypoxic Biological Activities of Rakicidin Derivatives on Human Pancreatic Cancer Cell Lines Panc-1, ASPC-1 and PATU8988
[0043] Make 2×10 cells to be tested 5 / mL cell suspension, add to 96-well plate round-bottom cell culture plate, add the compound to be tested respectively, 3 wells for each test concentration, place at 37°C, 5% CO 2 After culturing for 72 hours under saturated humidity conditions, the absorbance (A) value was measured at a wavelength of 570 nm in an enzyme-linked detector by the MTT method, and the inhibitory effect of the compound of the present invention on test cancer cells was calculated.
[0044] Table 1.Inhibitory activity of Rakicidin derivatives on various pancreatic cancer cells (IC 50 , μM)
[0045]
[0046] As shown in Table 1, the tested compounds showed strong anticancer activity against the tested cancer cell lines.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com