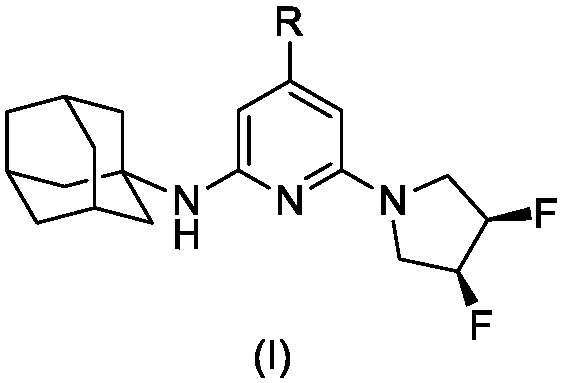
SSAO inhibitor containing adamantane structure as well as preparation method and application thereof
A technology of alkyl and cycloalkyl, applied in the field of SSAO inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1 Synthesis of Compound I-1
[0028]
[0029] Step 1. Synthesis of Compound IV-1
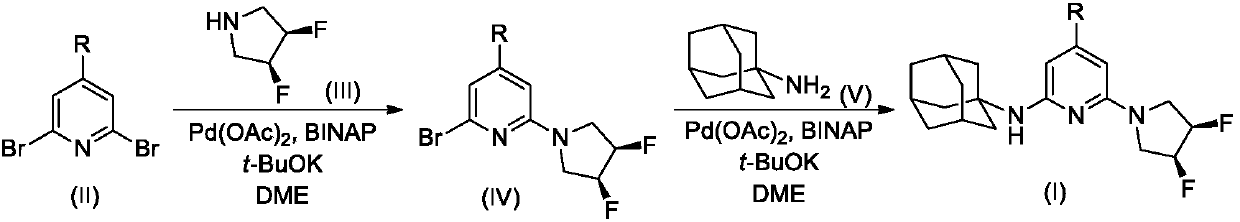
[0030] Compound II-1 (2.37g, 10mmol), Compound III (1.07g, 10mmol), Pd(OAc) 2 (0.22 g, 1 mmol), BINAP (2,2'-bisdiphenylphosphino-1,1'-binaphthyl, 0.62 g, 1 mmol) and t-BuOK (2.24 g, 20 mmol) were added to 50 mL of dry 1 , 2-dimethoxyethane (DME), the reaction mixture was stirred overnight under nitrogen atmosphere, and the reaction was found to be complete by TLC.
[0031] The reaction mixture was carefully poured into 200 mL of ice water, stirred, and washed with 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with 1% dilute hydrochloric acid and brine successively, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound IV-I, 1.76 g (yield 67%). ESI-MS, m / z=264(...
Embodiment 2
[0035] Example 2 Synthesis of Compound I-2
[0036]
[0037] Step 1. Synthesis of Compound IV-2
[0038] Compound II-2 (2.51g, 10mmol), Compound III (1.07g, 10mmol), Pd(OAc) 2 (0.22 g, 1 mmol), BINAP (2,2'-bisdiphenylphosphino-1,1'-binaphthyl, 0.62 g, 1 mmol) and t-BuOK (2.24 g, 20 mmol) were added to 50 mL of dry 1 , 2-dimethoxyethane (DME), the reaction mixture was stirred overnight under nitrogen atmosphere, and the reaction was found to be complete by TLC.
[0039] The reaction mixture was carefully poured into 200 mL of ice water, stirred, and washed with 50 mL × 3 CH 2 Cl 2 After extraction, the extract phases were combined, washed with 1% dilute hydrochloric acid and brine successively, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compound IV-2. ESI-MS, m / z=278([M+H] + ).
[004...
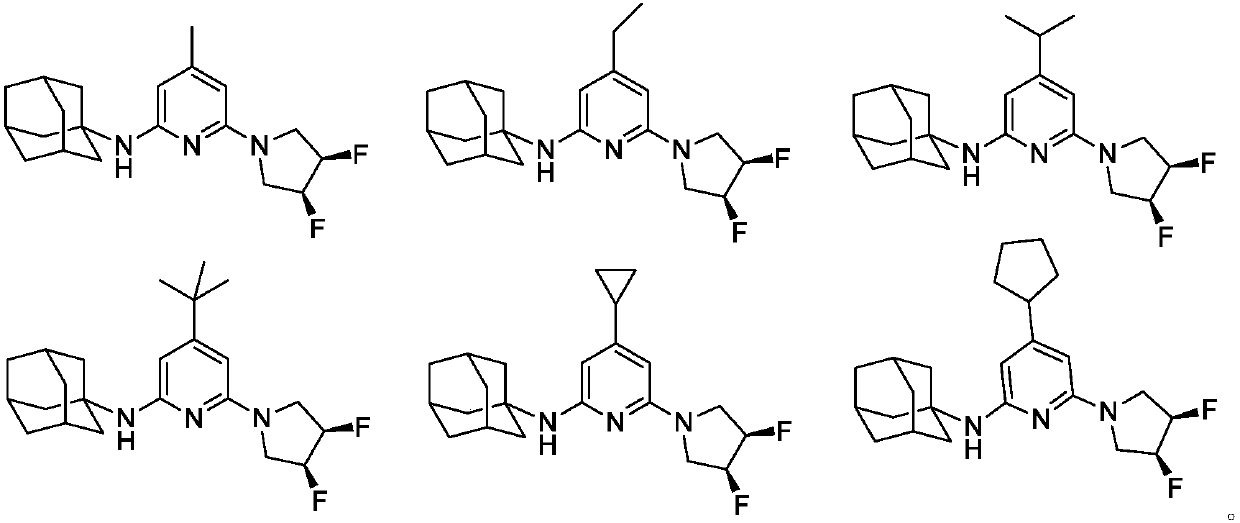
Embodiment 3-7
[0044] Referring to the methods of Examples 1 and 2, the following compounds were synthesized.
[0045]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com