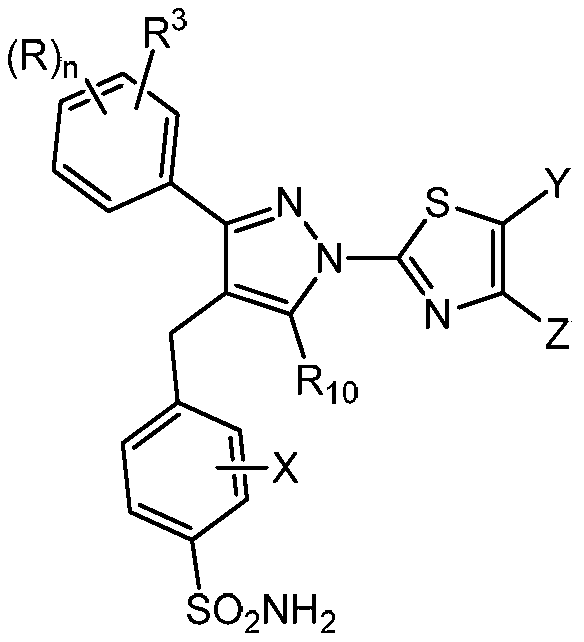
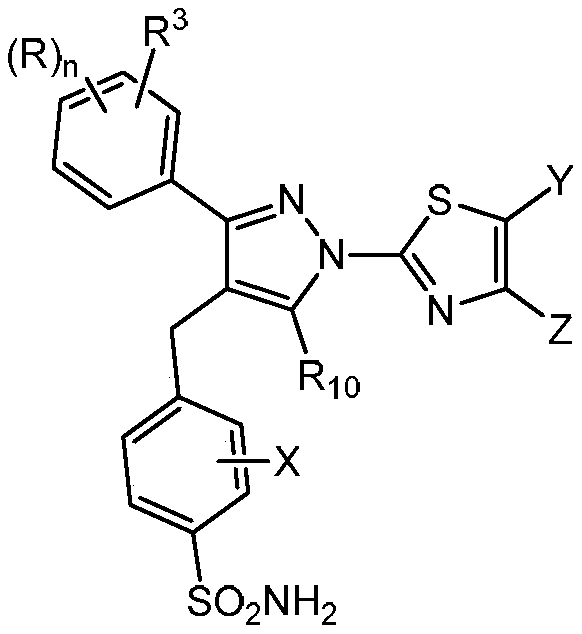
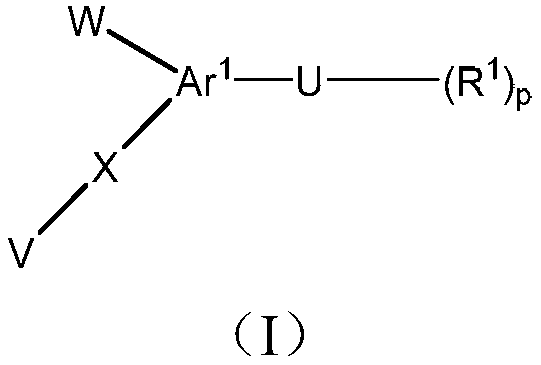
1h-pyrazol-1-yl-thiazoles as inhibitors of lactate dehydrogenase and methods of use thereof
A C1-C5, C1-C4 technology, applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, drug combinations, etc., can solve problems such as poor bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] This example describes the human LDHA primary biochemical assay used to characterize compounds of formula (I) in one embodiment of the invention.
[0114] Test compounds were placed in Greiner Bio-One (Monroe, NC) 1536-well black solid bottom assay plates. As assay buffer, use 200 millimolar (mM) Tris HCl, pH 7.4, 100 micromolar (μM) EDTA and 0.01% TWEEN-20 TM , the final concentration. The LDHA reagent was 2 nanomolar (nM) human LDHA (Meridian LifeScience, Inc., Memphis, TN), final concentration, in assay buffer. In assay buffer, substrate reagents were 0.06 mM NADH and 0.2 mM sodium pyruvate, final concentrations. The resazurin / diaphorase coupling reagent was 0.037 mM resazurin and 0.133 milligrams per milliliter (mg / mL) diaphorase in assay buffer, final concentration. The sequence of steps, the amount and type of reagents, and the time required for each step are set forth in Table 1. Inhibition of LDHA activity was measured by fluorescence emission.
[0115] Tab...
Embodiment 2
[0118] This example describes the human LDHB counterscreen biochemical assay used to characterize compounds of formula (I) in one embodiment of the invention.
[0119] Test compounds were placed in Greiner Bio-One (Monroe, NC) 1536-well black solid bottom assay plates. As assay buffer, use 200 mM Tris HCl, pH 7.4, 100 μM EDTA and 0.01% TWEEN-20 TM , the final concentration. LDHB reagent was 2 nM human LDHB (Meridian Life Science, Inc., Memphis, TN) at final concentration in assay buffer. In assay buffer, substrate reagents were 0.13 mM NADH and 0.16 mM sodium pyruvate, final concentrations. The resazurin / diaphorase coupling reagent was 0.037 mM resazurin and 0.133 mg / mL diaphorase in assay buffer, final concentration. The sequence of steps, the amount and type of reagents, and the time required for each step are set forth in Table 2. Inhibition of LDHB activity was measured by fluorescence emission.
[0120] Table 2
[0121]
Embodiment 3
[0123] This example describes the human PHGDH pair screening biochemical assay used to characterize compounds of formula (I) in one embodiment of the invention.
[0124] Test compounds were placed in Greiner Bio-One (Monroe, NC) 1536-well black solid bottom assay plates. As assay buffer, use 50 mM TEA, pH 8.0, 10 mM MgCl 2 , 0.05% BSA, and 0.01% TWEEN-20 TM , the final concentration. In assay buffer, substrate reagents were 10 μM EDTA, 0.625 mM glutamate, 500 nM human PSAT1, 500 nM human PSPH, 0.05 mM 3-phosphoglycerate, 0.1 mM resazurin, and 0.1 mg / mL diaphorase, final concentration. PHGDH reagent is 0.15 mM NAD in assay buffer + and 10 nM human PHGDH, final concentrations. The sequence of steps, the amount and type of reagents, and the time required for each step are set forth in Table 3. Inhibition of PHGDH activity was measured by fluorescence emission.
[0125] table 3
[0126]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com