Dental composition
A composition and dental technology, applied in dentistry, dental preparations, dental prostheses, etc., can solve problems such as high dynamic viscosity and low chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
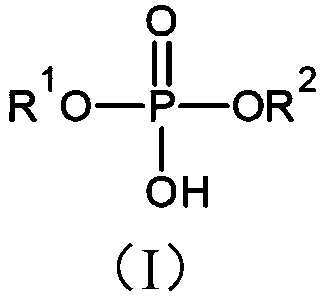
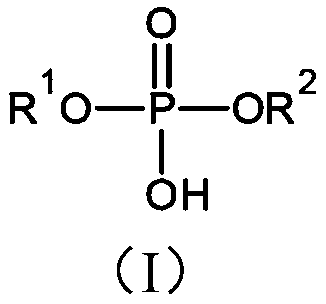
[0060] The preparation of phosphoric acid monoesters and diesters is generally well known and typical preparation routes are described for example in Houben-Weyl et al., Houben-Weyl Methods of Organic Chemistry, Volume XII / 2: Organic Chemistry Phosphorus Compounds II, 1964, pp. 143-210 and 226-274.
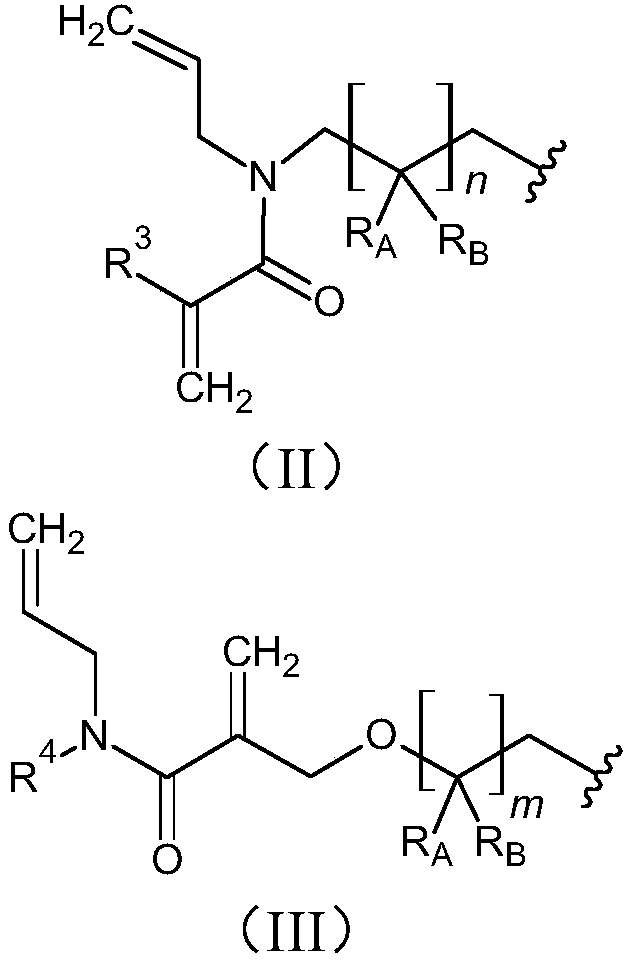
[0061] In particular, phosphoric acid monoester compounds of formula (I) can be prepared, for example, starting from precursor compounds of formula (VI), for introducing groups of formula (II) into compounds of formula (I). Precursor compounds of formula (VI) can be derived, for example, from allyl compounds of formula (V) obtained from compounds of formula (VI), as shown in Scheme 1:
[0062]
[0063] In compounds of formula (IV), (V) and (VI), n and R 3 has the same meaning as defined above for the group of formula (II) of the compound of formula (I), and R of the group of formula (II) A and R B Exemplarily represents a hydrogen atom.
[0064] For example, M. Porel et al....
example 1
[0406] Example 1: Preparation of N-acryloyl-8-allylamino-octyl phosphate
[0407] N-acryloyl-8-allylamino-octyl phosphate, the compound of formula (I), wherein R 1 is a group of formula (II), wherein R=hydrogen atom, and wherein n=6 and R 2 = Hydrogen atom, is prepared starting from octane diol in four steps as follows:
[0408] Step 1: Preparation of 8-bromo-octanol
[0409] 16 g (110 mmol) of octanediol were dissolved in 250 ml of toluene. After adding 15.5 ml of HBr (137 mmol, 1.25 equiv, 48% in water), the reaction mixture was refluxed with a dean-stark receiver to remove water from the reaction. After 8 hours, the mixture was cooled to room temperature, washed twice with distilled water and once with brine. After filtration over sodium sulfate and evaporation of the solvent, the bromide was obtained in quantitative yield. In the NMR spectrum, residual toluene was observed, which had no effect on subsequent steps.
[0410] D. 20℃ =1.23g / ml (document: 1.22g / ml)
[0...
example 2
[0423] N-Acryloyl-10-allylamino-decanol was prepared according to the synthesis described for N-acryloyl-8-allylamino-octanol.
[0424] N-Acryloyl-10-allylamino-decyl phosphate (ALP-10)
[0425] 20g (130mmol) POCl 3 Dissolve in 60 ml 2-methyl THF and cool with ice. Alcohol (30 g, 118 mmol) N-acryloyl-10-allylamino-decanol and NEt were added dropwise over a period of 45 minutes 3 (19ml, 118mmol) in 50ml 2-methyl THF. The mixture was stirred at room temperature for a further two hours, then 60 ml of water were added and the mixture was stirred for 30 minutes. The mixture was then transferred to a separatory funnel. The organic phase was washed once with water, then added to 80 ml 4N NaOH solution and stirred for 2 hours. The basic aqueous phase (containing the product) was separated and the organic phase was extracted once more with 80 ml of 2N NaOH solution. The combined aqueous phases were then acidified to pH 1 with concentrated HCl. Then 80 ml of 2-methyl THF were add...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com