Dihydromyricetin-berberine hydrochloride pharmaceutical co-crystals and preparation method thereof
A technology of berberine hydrochloride and dihydromyricetin, which is applied in the directions of drug combination, food science, anti-tumor drugs, etc., achieves the effects of anti-tumor effect, clear crystal structure, and simple and easy preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Suspend and stir 0.356 g of dihydromyricetin (dihydrate) and 0.407 g of berberine hydrochloride (dihydrate) in 20 mL of absolute ethanol for 24 hours, filter with suction, wash the resulting precipitate with absolute ethanol, and dry in the air; The obtained powder is recrystallized in absolute ethanol to obtain dihydromyricetin-berberine hydrochloride eutectic powder.
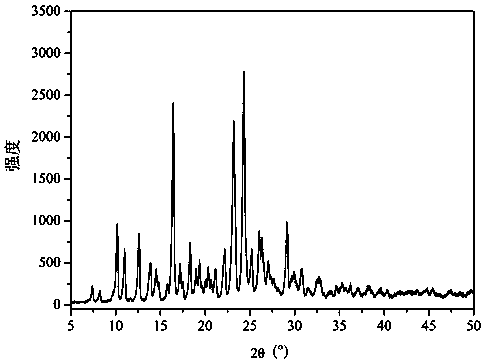
[0032] figure 1 It is the XRD figure of the dihydromyricetin-berberine hydrochloride cocrystal prepared in the present embodiment. Depend on figure 1 It can be seen that the prepared crystals are expressed as: 7.4°, 8.2°, 10.2°, 11.0,° 12.6°, 13.9°, 14.5°, 14.8°, 15.8°, 16.5°, 17.2°, 17.5°, 18.3°, 19.0°, 19.4°, 19.7°, 20.0°,20.4°, 20.7°, 21.2°, 22.2°, 23.2°, 24.3°, 25.2°, 26.0°, 26.4°, 27.1°, 27.4° , 27.6 °, 29.1 °, 29.7 °, 30.0 °, 30.8 °, 31.5 °, 32.6 ° have characteristic diffraction peaks.
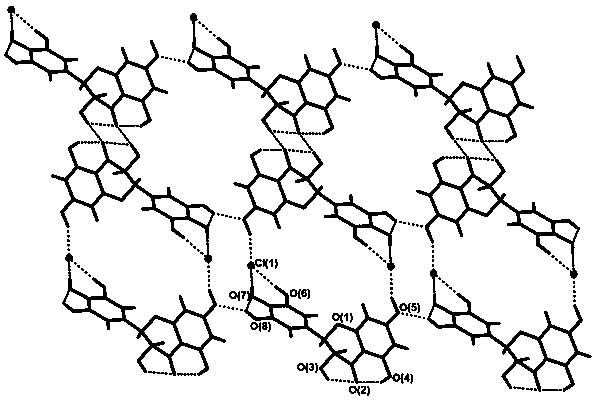
[0033] figure 2 It is the hydrogen bonding situation between dihydromyricetin and berberine hydrochlor...
Embodiment 2
[0037] Mix 0.356 g of dihydromyricetin (dihydrate) and 0.407 g of berberine hydrochloride (dihydrate) in a grinding jar, add 0.2 mL of absolute ethanol dropwise to moisten, and grind in a ball mill for 20 minutes to obtain dihydromyricetin Myricetin-berberine hydrochloride eutectic powder.
Embodiment 3
[0039] Dissolve 0.356 g of dihydromyricetin (dihydrate) and 0.407 g of berberine hydrochloride (dihydrate) in 100 mL of absolute ethanol under heating and stirring conditions, continue to stir after cooling, filter, and stand still to volatilize to obtain di Hydromyricetin-berberine hydrochloride eutectic crystal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com