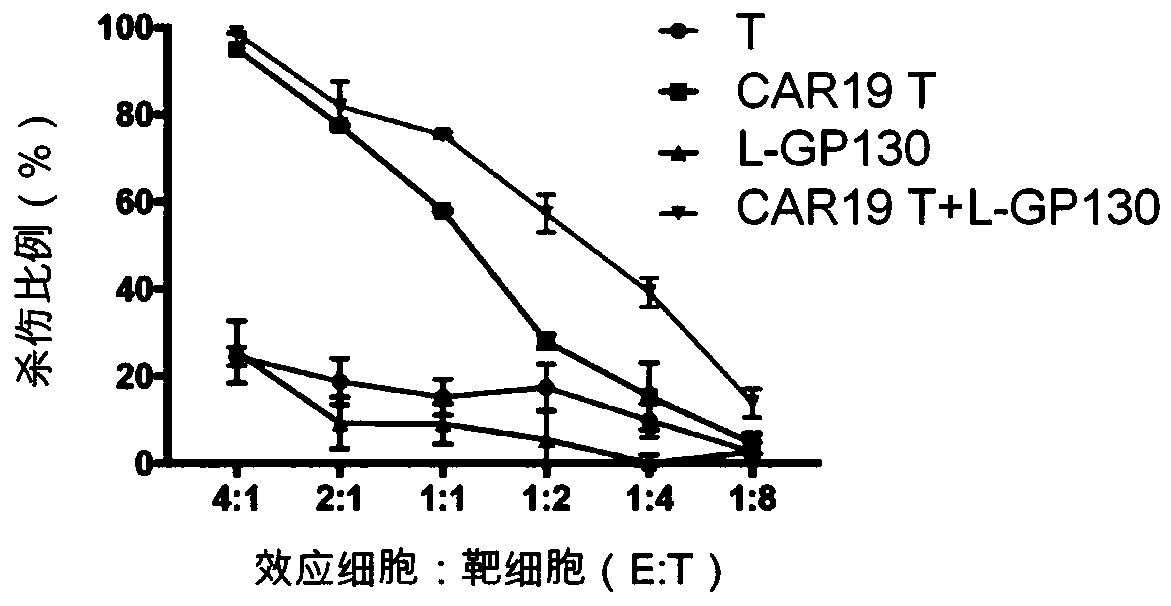
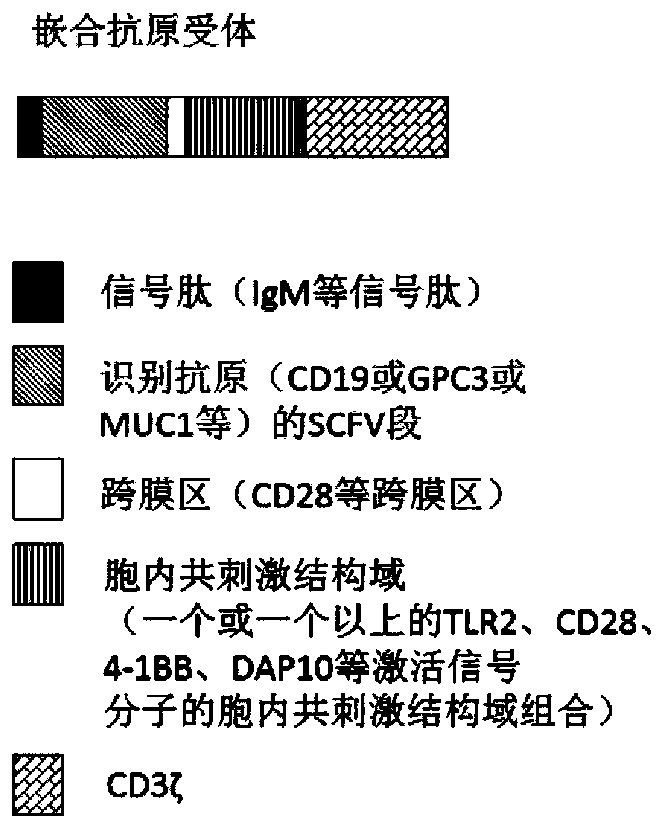
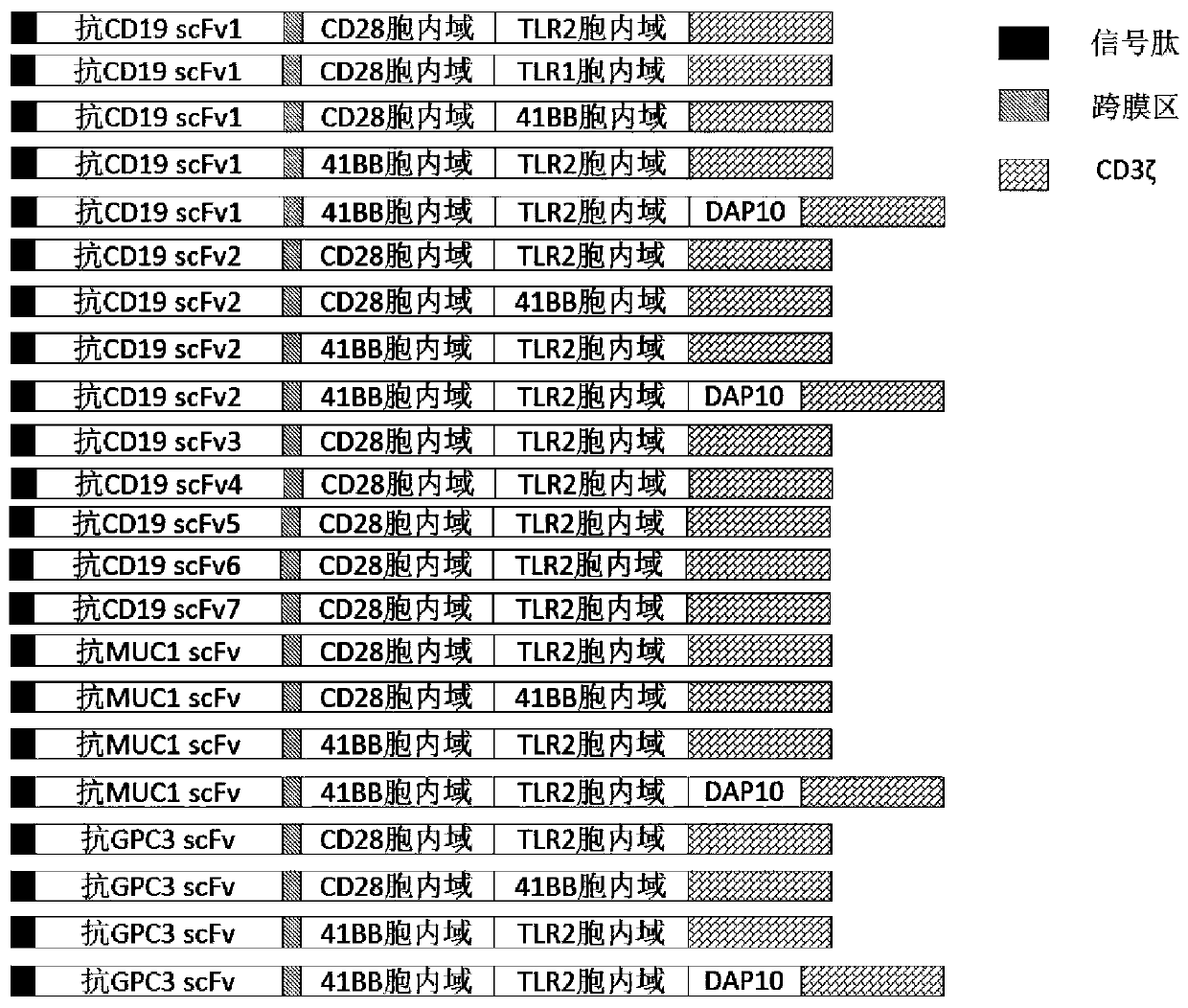
Genetically-modified immune cells and preparation method and application thereof
An immune cell and cell technology, applied in the field of immune cells overexpressing HIL-6 and/or L-GP130 and their preparation, can solve the problems of affecting the effect of anti-tumor treatment, affecting the anti-tumor effect of immune cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Constructing a persistent co-expression system
[0072] Through gene synthesis, the continuous co-expression plasmid vectors pUC57-CAR-2A-HIL-6 and pUC57-CAR-2A-L-GP130 of CAR molecules that recognize tumor antigens and HIL-6 or L-GP130 genes were obtained.
[0073] pUC57-CAR-2A-HIL-6 and pUC57-CAR-2A-L-GP130 were digested to obtain CAR-2A-HIL-6 and CAR-2A-L-GP130 genes, which were connected to the lentiviral vector pWPXLd (containing GFP gene) or PB vector (containing GFP gene), construct pWPXLd-CAR-2A-HIL-6-2A-GFP and pWPXLd-CAR-2A-L-GP130-2A-GFP, or PB-CAR-2A-HIL -6-2A-GFP and PB-CAR-2A-L-GP130-2A-GFP.
[0074] In this embodiment, the amino acid sequence of HIL-6 is SEQ ID NO: 1 or an amino acid sequence having the same or similar activity with more than 80% homology with SEQ ID NO: 1, and the nucleic acid sequence is as shown in SEQ ID NO: 2 The amino acid sequence of L-GP130 is SEQ ID NO: 3 or an amino acid sequence having the same or similar activity w...
Embodiment 2
[0081] Example 2 Constructing a continuous separate co-expression system
[0082] Through molecular cloning, plasmid vectors pUC57-CAR, pUC57-HIL-6, and pUC57-L-GP130, which continuously express CAR molecules that recognize tumor antigens, HIL-6 gene, and L-GP130 gene, were obtained. Digest pUC57-CAR, pUC57-HIL-6 and pUC57-L-GP130 to obtain CAR, HIL-6 and L-GP130 genes, and connect them to lentiviral vector pWPXLd (containing GFP gene) or PB vector (containing GFP gene ), construct pWPXLd-CAR-2A-GFP, pWPXLd-HIL-6-2A-GFP and pWPXLd-L-GP130-2A-GFP, or PB-CAR-2A-GFP, PB-HIL-6-2A-GFP and PB-L-GP130-2A-GFP.
[0083] In this example, the sequence information of the CAR molecule, HIL-6, L-GP130 and vector used in the construction system is the same as that in Example 1.
Embodiment 3
[0084] Example 3 Constructing CAR recognition and activation-induced overexpression system
[0085] Through gene synthesis, the plasmid vector pUC57-CAR expressing the CAR molecule recognizing tumor antigen, NFAT repeat sequence (6 repeat sequences)-IL2 promoter-HIL-6 gene and NFAT repeat sequence-IL2 promoter-L-GP130 gene was obtained , pUC57-NFAT repeat-IL2 promoter-HIL-6 and pUC57-NFAT repeat-IL2 promoter-L-GP130. Digest pUC57-CAR, pUC57-NFAT repeat-IL2 promoter-HIL-6 and pUC57-NFAT repeat-IL2 promoter-L-GP130 to obtain CAR, NFAT repeat-IL2 promoter-HIL-6 and NFAT repeat sequence-IL2 promoter-L-GP130 gene, connected to lentiviral vector pWPXLd (no promoter, containing GFP gene) or PB vector (no promoter, containing GFP gene), construct pWPXLd-CAR-2A- GFP, pWPXLd(no promoter)-NFAT repeat-IL2 promoter-HIL-6-2A-GFP and pWPXLd(no promoter)-NFAT repeat-IL2 promoter-L-GP130-2A-GFP, or PB -CAR-2A-GFP, PB(no promoter)-NFAT repeat-IL2 promoter-HIL-6-2A-GFP and PB(no promoter)-NFAT...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com