A kind of cabazitaxel protein nano-injection and preparation method thereof
A nano-injection, cabazitaxel technology, applied in the field of pharmaceutical preparations, can solve the problems of long time, large dosage, large toxic and side effects, hypersensitivity reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Cabazitaxel protein nano-injection, including the following raw materials:
[0038] Cabazitaxel 100mg; Cholesterol 150mg; Bovine serum albumin 1.5g (1.5% concentration); Sodium octanoate 12mg.
[0039] making process:
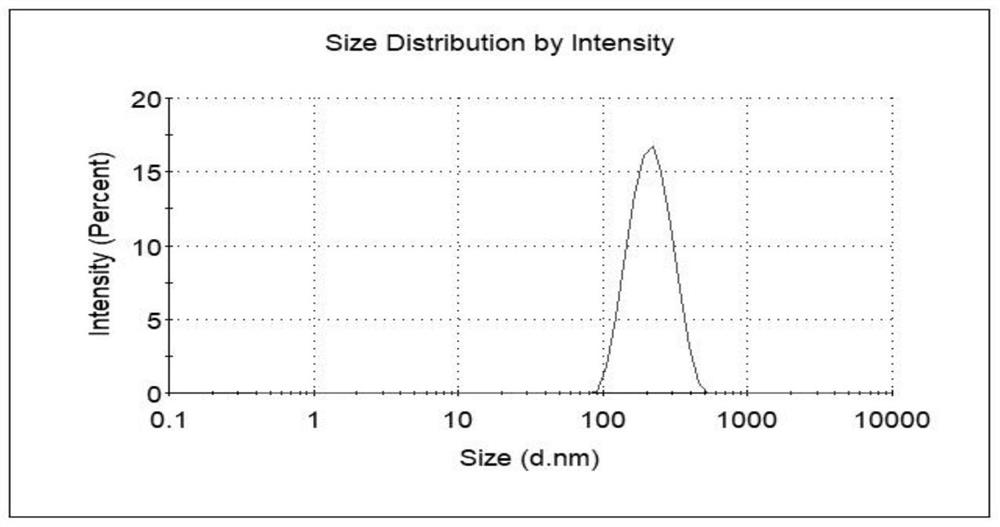
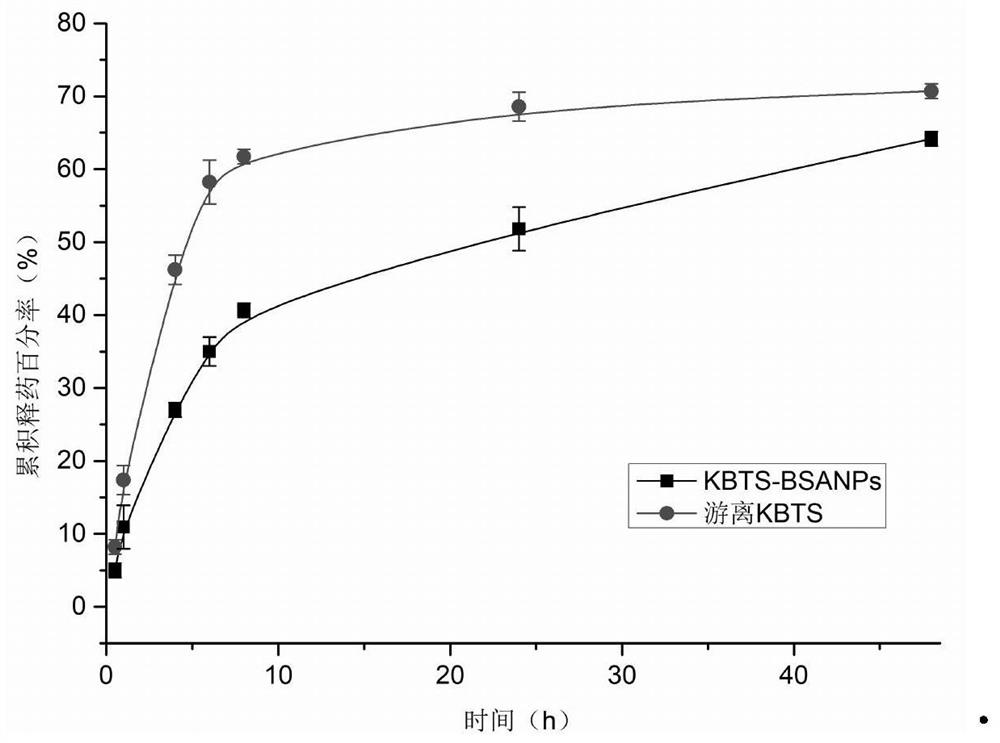
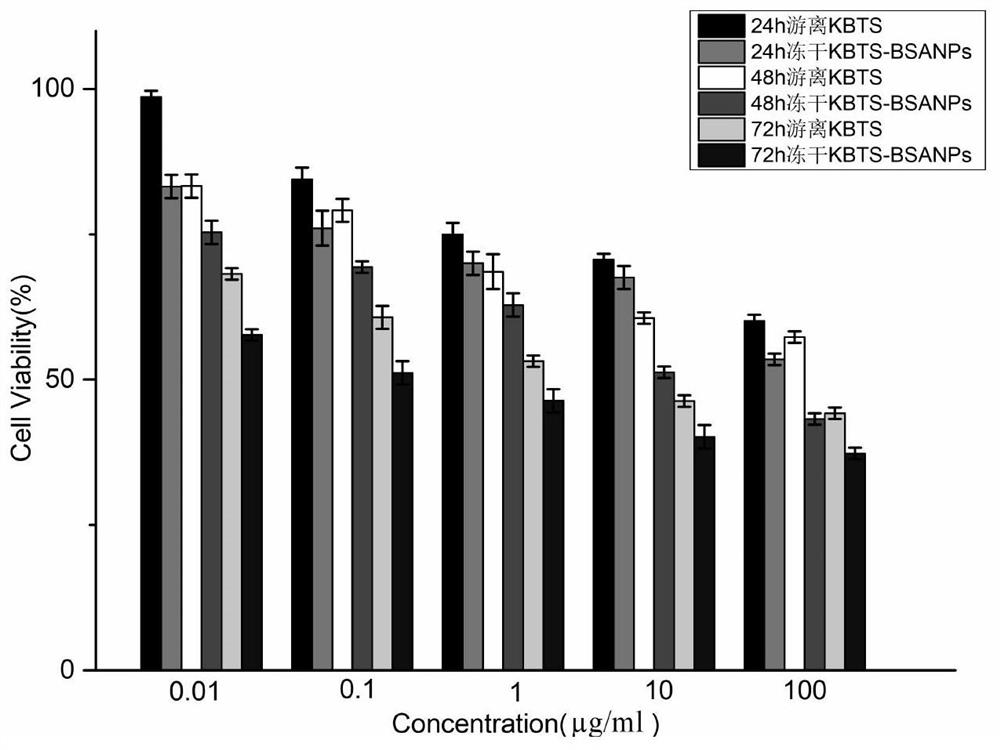
[0040]Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve bovine serum albumin in ultrapure water, adjust the pH to 10, Standby as the water phase; add the oil phase to the water phase, and perform ultrasonication in an ice bath at 30% power for 12 minutes to form a primary emulsion; pass the primary emulsion through a high-pressure homogenizer at a pressure of 800 bar, and circulate it 8 times The treated emulsion is subjected to 35°C decompression rotary steaming process to remove the organic solvent, centrifuged at 22000r / min (34700×g) for 30min at 4°C, removes the supernatant, and adds an appropriate amount of ultrapure water to the precip...
Embodiment 2
[0042] Cabazitaxel protein nano-injection, including the following raw materials:
[0043] Cabazitaxel 150mg; Cholesterol 200mg; Bovine serum albumin 1.5g (1.5% concentration); Sodium caprylate 12mg.
[0044] making process:
[0045] Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve bovine serum albumin in ultrapure water, adjust the pH to 8, Standby as the water phase; add the oil phase into the water phase, and perform ultrasonication in an ice bath at 40% power for 8 minutes to form a primary emulsion; pass the primary emulsion through a high-pressure homogenizer under the condition of a pressure of 500 bar, and circulate 8 times ; The processed emulsion was subjected to vacuum rotary evaporation at 35°C to remove the organic solvent, centrifuged at 22,000 r / min (34,700×g) and 4°C for 30 minutes, removed the supernatant, and reconstituted the precipitate with a...
Embodiment 3
[0047] Cabazitaxel protein nano-injection, including the following raw materials:
[0048] Cabazitaxel 100mg; Cholesterol 100mg; Human serum albumin 2.0g (2% concentration); Sodium caprylate 16mg.
[0049] making process:
[0050] Dissolve cabazitaxel and cholesterol in an organic solvent (chloroform: absolute ethanol = 11:1, 10ml in total), and use it as an oil phase for later use; dissolve human serum albumin in ultrapure water, adjust the pH to 8, Standby as the water phase; add the oil phase into the water phase, and perform ultrasonication in an ice bath at 40% power for 3 minutes to form a primary emulsion; continue stirring the primary emulsion at room temperature for 2 hours; Press and spin steam to remove the organic solvent, centrifuge at 22000r / min (34700×g) for 30min at 4°C, remove the supernatant, and redissolve the precipitate with an appropriate amount of ultrapure water to obtain the cabazitaxel protein nanomaterial; the cabazitaxel Sai protein nanomaterials ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com