Non-self-cutting trypsin and preparation method thereof
A technology of trypsin and porcine trypsin, applied in the direction of biochemical equipment and methods, enzymes, peptidases, etc., can solve problems such as instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Embodiment 1, the mutation analysis of porcine trypsin (Pt)
[0085] Porcine trypsin (Pt) is prone to autodegradation. Its amino acid sequence is as follows (SEQ ID NO: 1):
[0086] IVGGYTCAANSIPYQVSLNSGSHFCGGSLINSQWVVVSAAHCYKSRIQV LGEHNIDVLEGNEQFINAAKIITHPNFNGNTLDNDIMLIKLSSPATLNS VATVSLP CAAAGTECLISGWGNTKSSGSSYPSLLQCLKAPVLSDSSCKSSYPGQITGNMICVGFLEGGKDSCQGDSGGPVVCNGQLQGIVSWGYGCAQKNKPGVYTKVCNYVNWIQQTIAAN
[0087] The present inventors studied the stability of wild-type Pt, and analyzed its self-degrading short peptide, and found its most important self-degrading fragment. The amino acid positions corresponding to these self-degrading fragments are Arg49, Arg99, and Arg107, respectively. Among them, Arg49 and Arg107 may be the secondary degradation sites of Pt.
[0088] The present inventors used the method of site-directed mutagenesis to study the effect of the mutation at position 99 on the stability, including Pt-R99L (mutation of Arg to Leu) and Pt-R99H (mutat...
Embodiment 2
[0091] Embodiment 2, the effect of Arg99 site on stability
[0092] In Example 1, the inventors designed and recombinantly expressed rPt, rPt-R99L (PTL), rPt-R99H (PTH), purified them, and studied their stability.
[0093] 1. Temperature stability
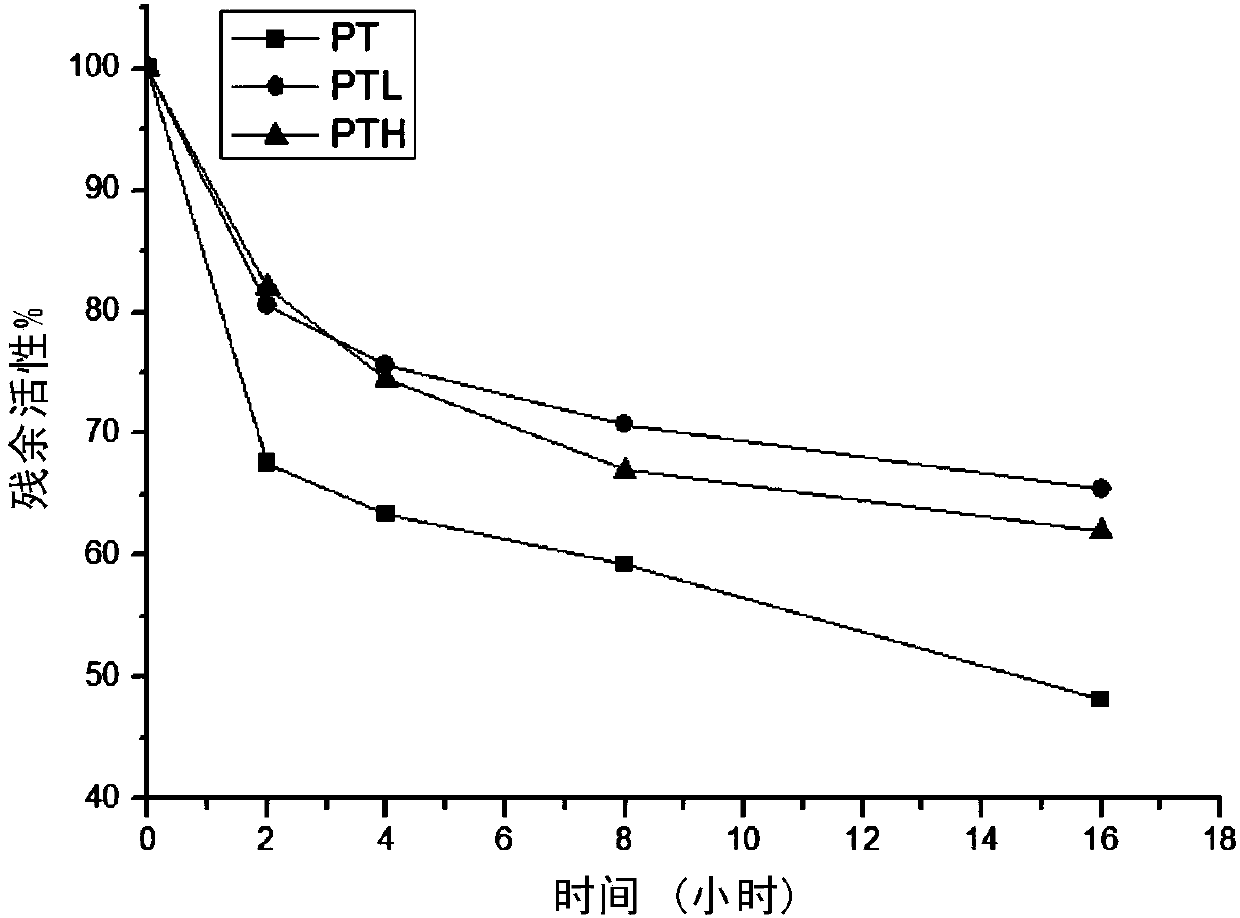
[0094] The inventors compared the stability of rPt, rPt-R99L, and rPt-R99H at 60°C. The enzymes were preserved in 2mM hydrochloric acid and then incubated in a 60°C water bath, and the activity was measured at regular intervals.
[0095] The result is as Figure 1A shown. It can be seen from the figure that the activities of the three decreased the fastest in the first 2 hours, rPt decreased by 35%, rPt-R99L and rPt-R99H decreased by 20% and 18% respectively, and the activity of the three decreased slowly after 2 hours, but they could It is obvious that the overall activity of rPt-R99L and rPt-R99H is 15%-20% higher than that of wild-type rPt, and the thermal stability of rPt-R99L is about 5% higher than that of rPt-R99H.
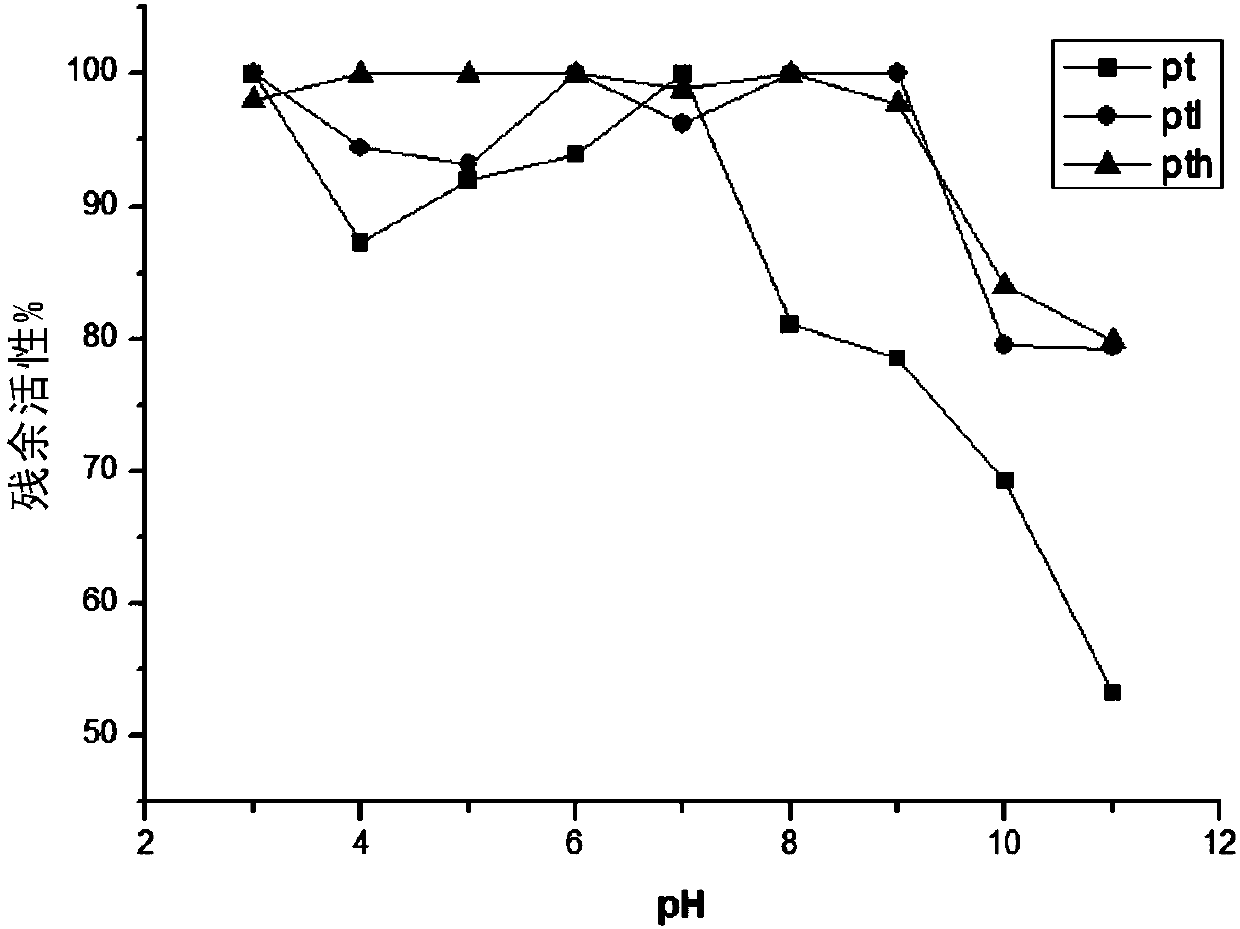
[009...
Embodiment 3
[0107] The stability of the mutant further mutated on the basis of embodiment 3, rPt-R99H
[0108] 1. Temperature stability
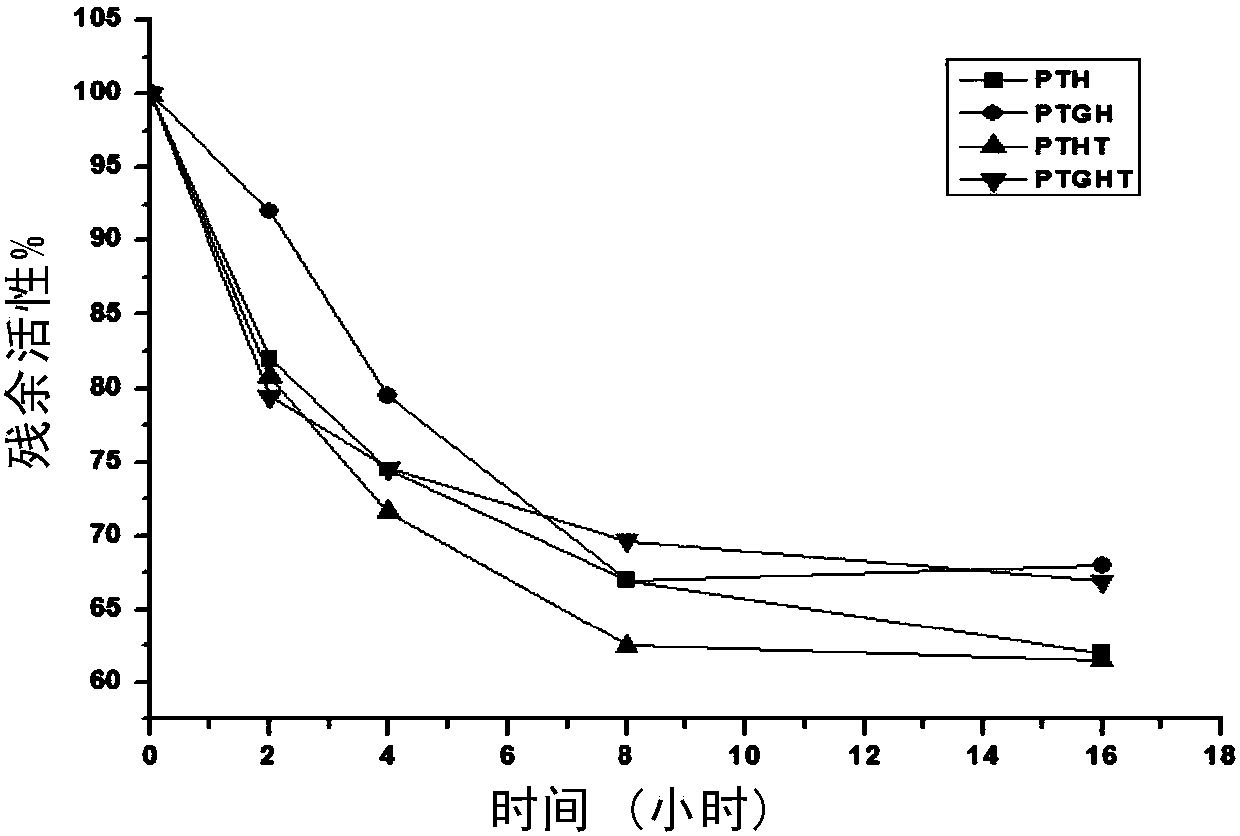
[0109] In the present embodiment, porcine trypsin (rPt) and mutant thereof are divided into following three kinds:
[0110] PTH: rPt-R99H;
[0111] PTGH: rPt-R49G-R99H;
[0112] PTHT: rPt-R99H-R107T;
[0113] PTGHT: rPT-triple mutation, ie rPt-R49G-R99H-R107T.
[0114] Synthesize the above enzyme mutants, dilute the purified above four enzymes to 0.5mg / ml, then incubate them in a water bath at 60°C, measure their activity at 0h, 2h, 4h, 8h, and 16h, and take the activity at 0h As 100%, calculate the activity residual rate at other times, and make a graph to show the relationship between time and activity residual rate.
[0115] The result is as figure 2, Comparing the stability of the four mutants at 60°C, it can be seen that the rPt-R49G-R99H, rPt-R99H-R107T, and rPT-triple mutant mutants constructed on the basis of rPt-R99H are rPt-R99H, rPt-R9...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com