A kind of preparation method of dithiophenophthalimide
A technology of dithiophenophthalimide and phenophthalimide, which is applied in the field of organic compound synthesis, can solve the problems of less research on the application of dithiophenophthalimide, low yield, and severe toxicity. and other problems, to achieve the effect of high yield, no highly toxic substances, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 【Reaction route】
[0024]
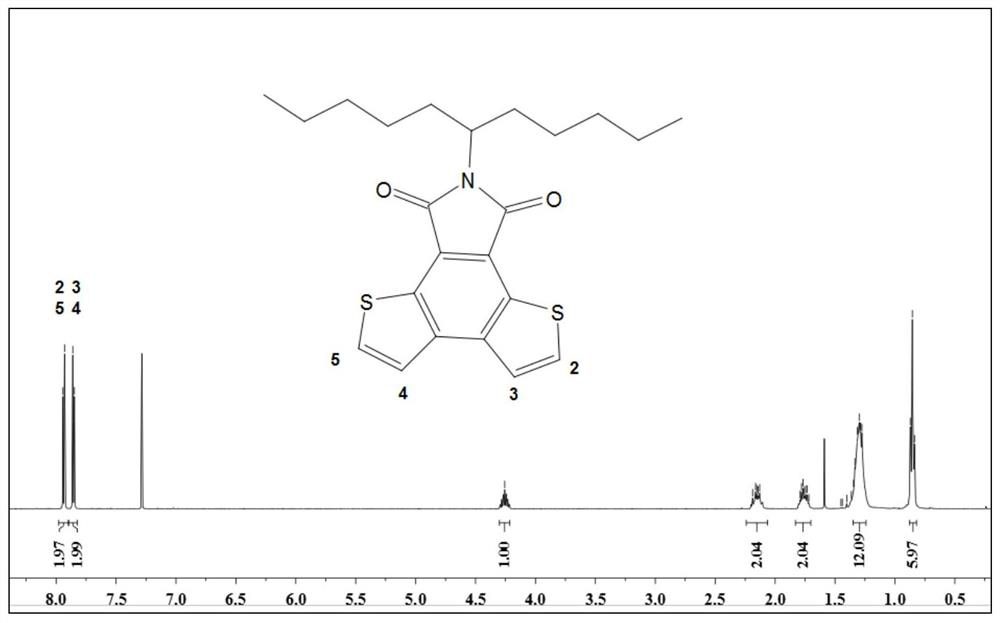
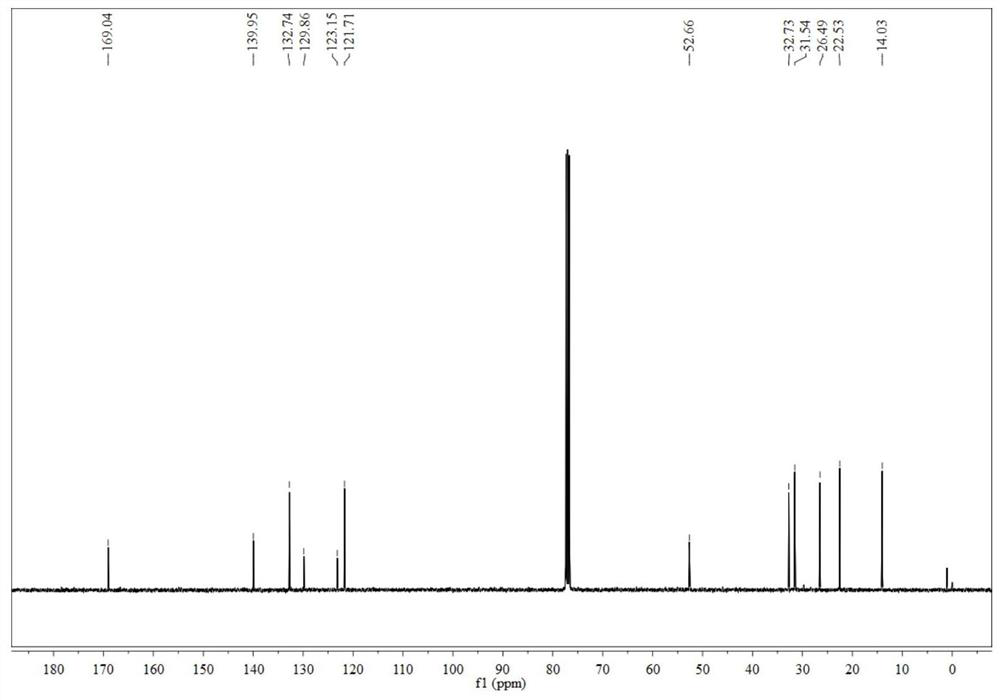
[0025] According to the reaction route, 2-thiopheneglyoxylic acid (0.5g, 3.2mmol) and potassium hydroxide (196mg, 3.5mmol) were dissolved in 20mL of aqueous solution, the temperature was controlled at 100°C, the mixture was stirred and heated under reflux for 10h, and the aqueous solution was spin-dried after the reaction was completed. Product B is obtained. 1 HNMR (400MHz, DMSO-d6, δ, ppm): 7.89 (dd, J = 4.9, 0.8Hz, 1H), 7.71 (dd, J = 3.7, 0.8Hz, 1H), 7.17 (dd, J = 4.7, 3.9 Hz,1H).
Embodiment 2
[0027] 【Reaction route】
[0028]
[0029] Dissolve product B (100mg, 0.52mmol) and thiophene acetate (80mg, 0.6mmol) in 10mL of acetic anhydride solvent, control the temperature at 120°C, reflux, stir and heat for 12h, slowly pour into water after the reaction, and extract with saturated sodium chloride / After water, 100-200 mesh silica gel was used as the stationary phase, and petroleum ether / dichloromethane (volume ratio 3:1) was used as the eluent to pass through the column, and the product D was obtained after purification.
Embodiment 3
[0031] 【Reaction route】
[0032]
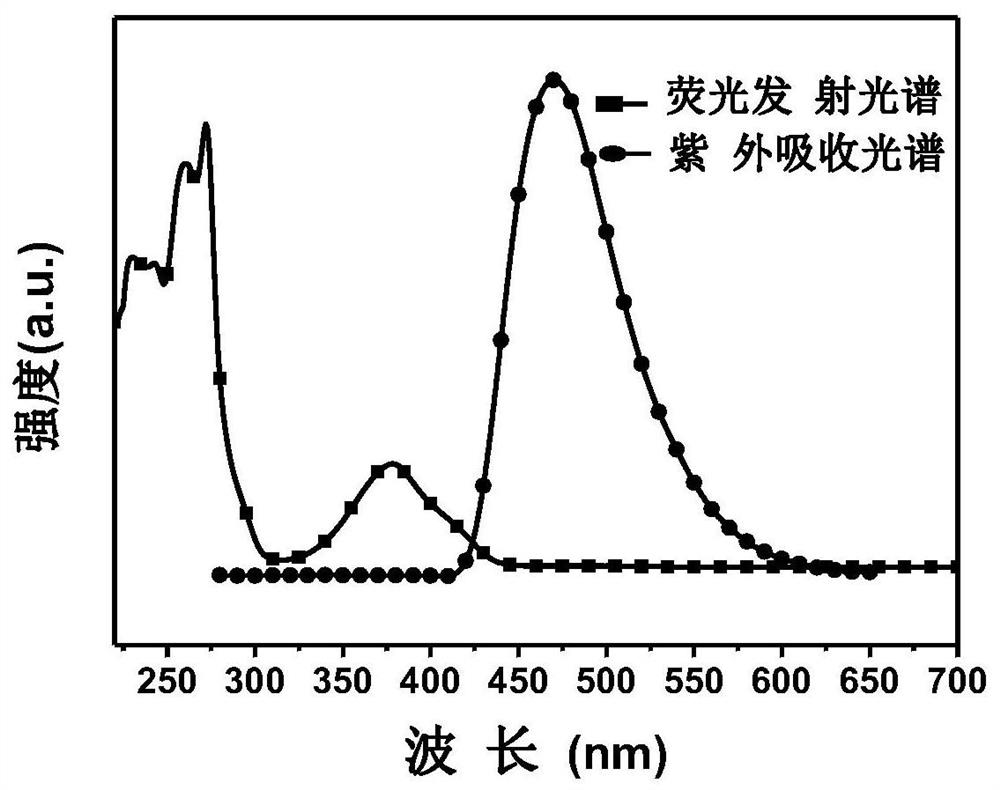
[0033] According to the reaction route, the product D (1g, 3.8mmol) was dissolved in 200mL of dichloromethane solution, and elemental iodine (100mg, 0.38mmol) was added as a catalyst. Mesh silica gel was used as the stationary phase, and petroleum ether / dichloromethane (system ratio 5:1) was used as the eluent to pass through the column for purification to obtain fused-ring dithiophene anhydride (E).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com