A kind of ω-transaminase double mutant and its application
A double mutation, transaminase technology, applied in the field of molecular biology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
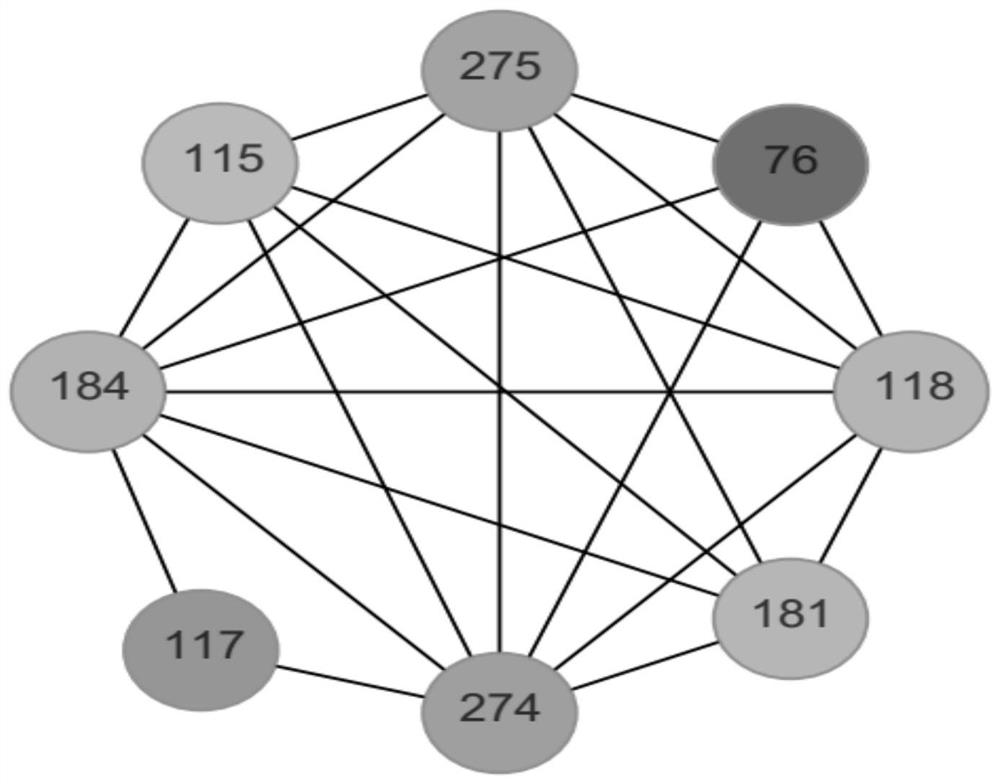
[0034] 1. Co-evolution network prediction
[0035] Protein coevolution is of great significance for gaining insight into the action process and protein structure of functional proteins. Through the measurement of protein co-evolution, residue sites that play an important role in protein structure and function can be found.
[0036] Specific steps are as follows:
[0037] (1) The PDB file (PDB ID: 4CE5) of Aspergillus terreus (Aspergillus terreus) ω-transaminase was compared in the NCBI database (https: / / www.ncbi.nlm.nih.gov / ) through BLASTP multiple sequence alignment to obtain Related protein family number pfam:01063.
[0038] (2) Based on mutual information (Mutual Information, MI), through different biological characteristics, the protein co-evolution website MISTIC (Mutual Information Server To Infer Coevolution) is used to infer protein coevolution through mutual information, and an amino acid residue co-evolution network is generated;
[0039]Upload the protein family...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com