Compounds useful for the treatment and/or care of the skin, hair, nails and/or mucous membranes
A compound, AA1-AA6 technology, used in skin care preparations, skin diseases, receptors/cell surface antigens/cell surface determinants, etc., can solve problems such as skin microbiota imbalance, and achieve the goal of improving physical and immune barriers. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0262] The preparation method of the compound of the present invention
[0263] The synthesis of the compounds of the present invention, their stereoisomers, their mixtures and / or their cosmetically or pharmaceutically acceptable salts can be carried out according to conventional methods known in the art, such as solid-phase peptide synthesis methods [Stewart J.M. and Young J. .D., "Solid Phase Peptide Synthesis, 2nd edition", (1984), Pierce Chemical Company, Rockford, Illinois; Bodanzsky M. and Bodanzsky A., "The practice of Peptide Synthesis", (1994), Springer Verlag, Berlin; Lloyd -Williams P. et al., "Chemical Approaches to the Synthesis of Peptides and Proteins", (1997), CRC, Boca Raton, FL, USA], synthesis in solution, enzymatic synthesis [Kullmann W. "Proteases as catalysts for enzymic syntheses of opioid peptides”, (1980), J.Biol.Chem., 255(17), 8234-8238] or any combination thereof. The compound can also be obtained by fermentation of bacterial strains, modified or ...
Embodiment 1
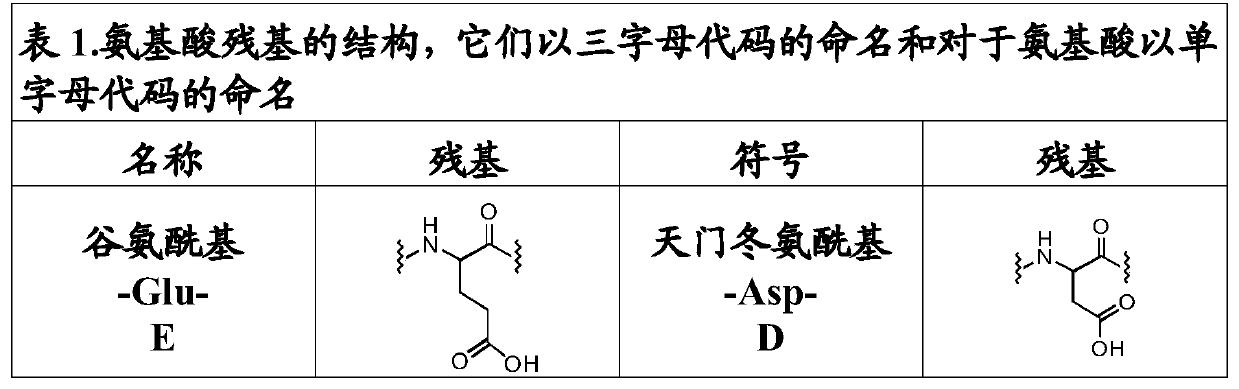
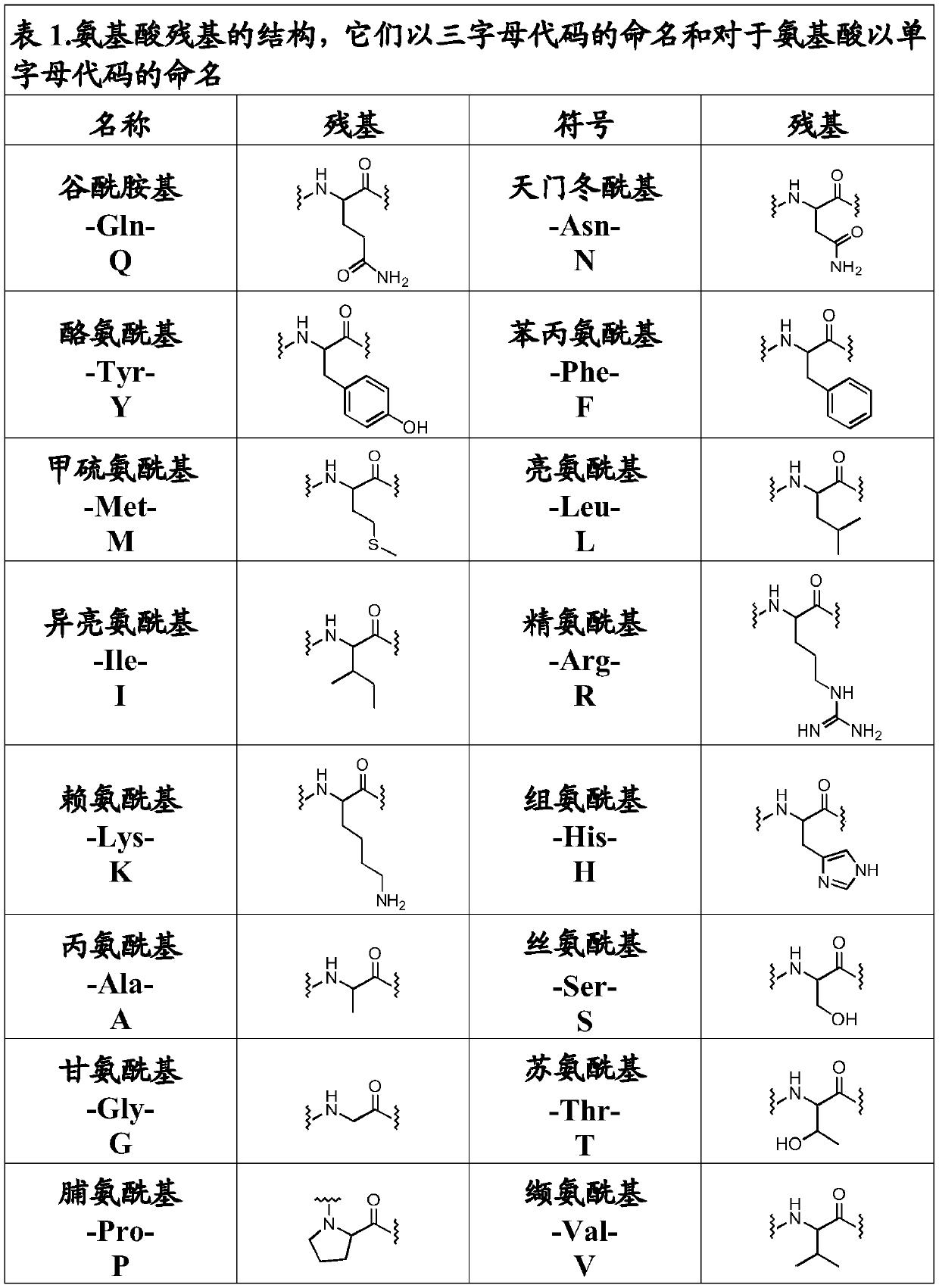
[0358] Get H-AA 8 -O-2-ClTrt-(R), where AA 8 is L-Asp, L-Asn or L-Glu.
[0359]Weights have been normalized. Dissolve 3.2mmol (1 equivalent) of Fmoc-L-Asp(tBu)-OH, Fmoc-L-Asn(Trt)-OH or Fmoc-L-Glu(tBu)-OH in 20ml of DCM, add 0.83 Equivalents of DIEA, coupled to dry 2-chlorotrityl resin (3.2 mmol), functionalized at 1.6 mmol / g. The mixture was stirred for 5 minutes, then 1.63 equivalents of DIEA were added. The mixture was allowed to react for 40 minutes. The remaining chloride groups were blocked by treatment with 2 ml of MeOH.
[0360] The resin was then washed and the N-terminal Fmoc group deprotected as described in the general methods.
[0361] Get H-AA 6 -O-2-ClTrt-(R) where AA 6 is L-Arg or L-Lys.
[0362] Weights have been normalized. 1.6 mmol (1 equiv) of Fmoc-Arg(Pbf)-OH or Fmoc-L-Lys(Boc)-OH dissolved in 10 ml of DCM, to which 0.83 equiv of DIEA was added, was coupled to dry 2-chlorotrityl On base resin (1.6 mmol), the functionalization was 1.6 mmol / g. Th...
Embodiment 2
[0365] Get Fmoc-W m -X n -AA 1 -AA 2 -AA 3- AAA 4 -AA 5 -AA 6 -AA 7 -AA 8 -Y p -Z q -O-2-ClTrt-(R), where AA 1 is L-Glu; AA 2 is L-Glu; AA 3 is L-Met; AA 4 is L-Gln; AA 5 is L-Arg; AA 6 is L-Arg; AA 7 Is Ala; AA 8 is L-Asp; n, m, p and q are each 0.
[0366] Weights have been normalized. 143 mg of H-L-Asp(tBu)-O-2-Cl-Trt resin with 0.98 mmol / g (0.14 mmol) functionalization were washed as described in General Methods. Following the described protocol, 5 equivalents of Fmoc-Ala-OH (Fmoc-AA 7 -OH) was coupled to the peptidyl resin for 60 minutes.
[0367] The resin was then washed as described in the general method, and the deprotection treatment of the Fmoc group was repeated for coupling of the next amino acid. Following the previously described protocol, 5 equivalents of Fmoc-L-Arg(Pbf)-OH(Fmoc-AA 6 -OH) was coupled to the peptidyl resin for 60 minutes. The resin was then washed as described in the general method, and the deprotection treatment of the F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com