Modified nanopores, compositions comprising the same, and uses thereof
A technology of nanopore and pancreatic juice, applied in the direction of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0296] Example 1: For expression and purification of modified secretin nanopore subunit polypeptides such as modified InvG nano Exemplary Methods of Pore Subunit Polypeptides
[0297] A secretin nanopore subunit polypeptide (for example, amino acid sequence as shown in SEQ ID NO: 1 or 2 containing coding modification, which has one or more (for example, 1, 2, 3, 4, 5, 6, 7 , 8, 9, 10, 15, 20, 25, 30 or more and up to 50 of the amino acid modifications described herein)) of the ampicillin-resistant pT7 vector with a C-terminal hexahistidine (His) tag C43DE3pLysS cells were cotransformed with a kanamycin-resistant pRham vector containing a gene encoding InvH protein (a 20Kd protein that will enhance InvG expression), and in the presence of ampicillin (100 μg / ml) and kanamycin (30 μg / ml ml) agar plates and grown overnight at 37°C. A single colony was used to inoculate a 100 ml starter culture of TB medium containing ampicillin (100 μg / ml) and kanamycin (30 μg / ml). The cultu...
Embodiment 2
[0299] Example 2 for expression and purification of a modified secretin nanopore subunit polypeptide comprising an endopeptidase cleavage site Exemplary method for
[0300]Ampicillin-resistant pT7 vector containing a gene encoding a modified secretin nanopore subunit polypeptide - which contains an endopeptidase cleavage site, such as tobacco etch virus (TEV) protease cleavage site - with a C-terminal hexahistidine tag position (for example, the amino acid sequence shown in SEQ ID NO: 3, which has one or more (for example, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25 , 30 or more and up to 50 amino acid modifications described herein)) - transformed into C43DE3pLysS cells and plated on agar plates containing ampicillin (100 μg / ml). A single colony was used to inoculate a 100 ml starter culture of TB medium containing ampicillin (100 μg / ml). The culture was stirred at 250 rpm for 18 hours at 37°C. 15 ml starter culture was used to inoculate 500 ml TB medium containing ampici...
Embodiment 3
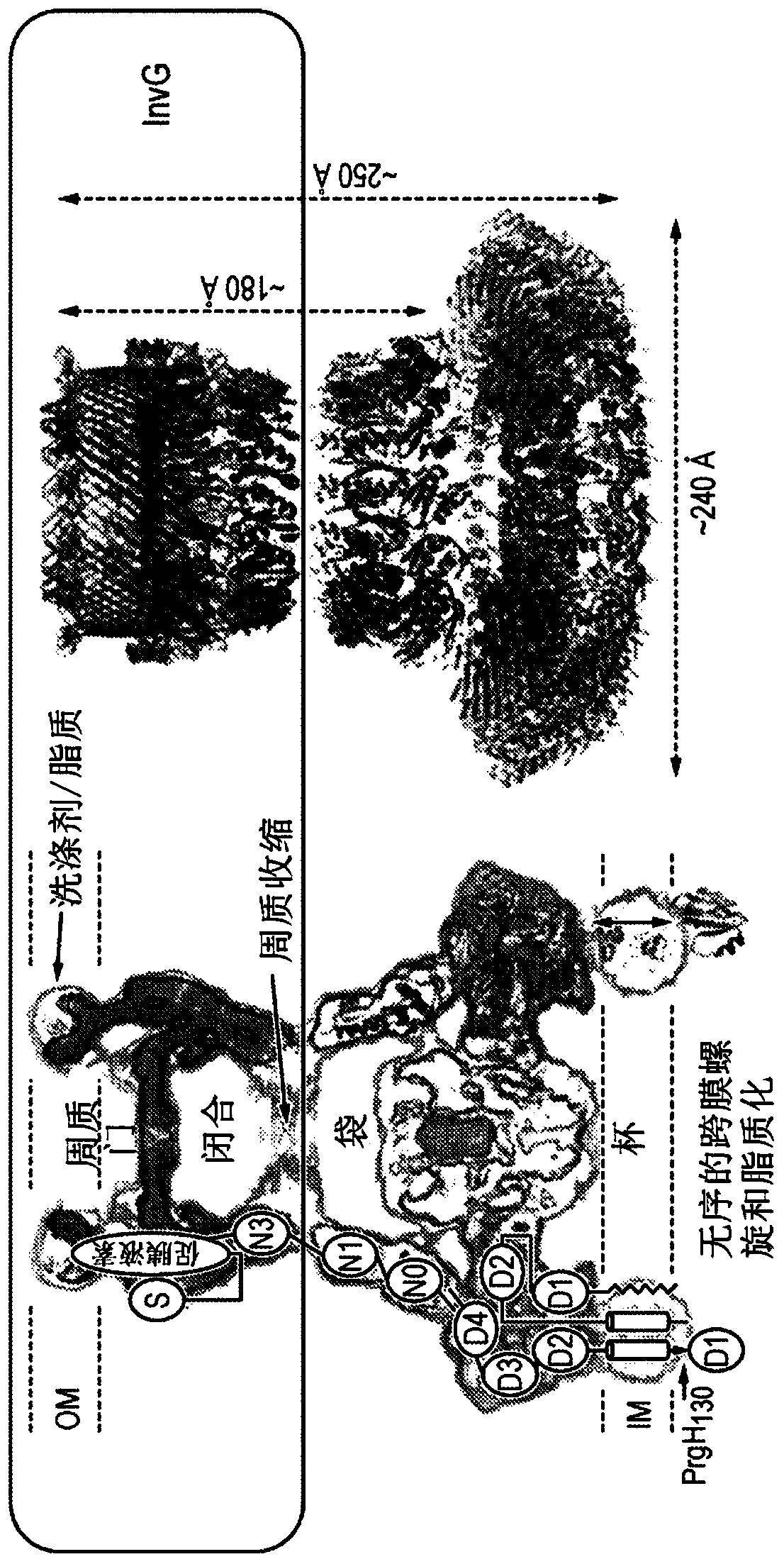
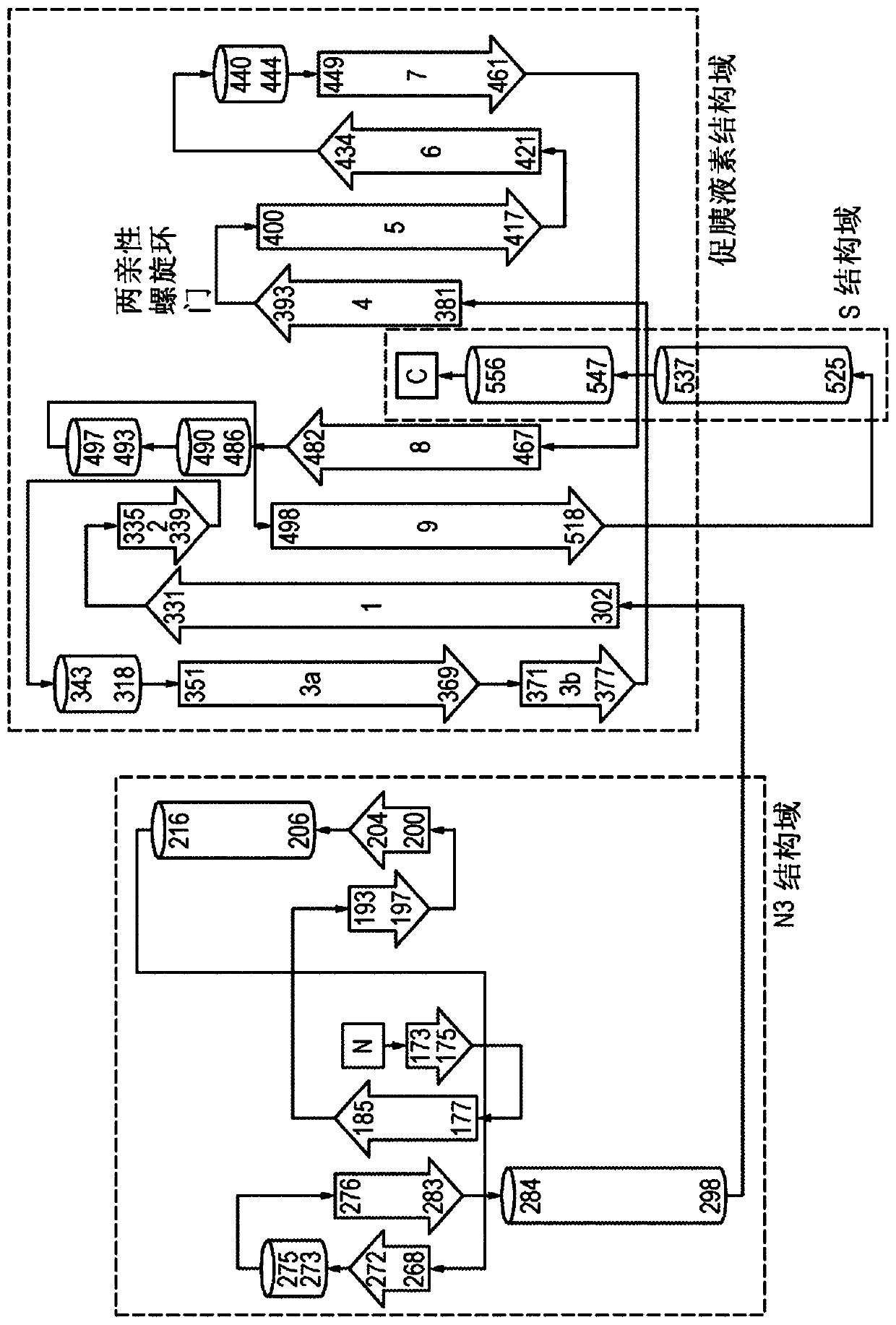
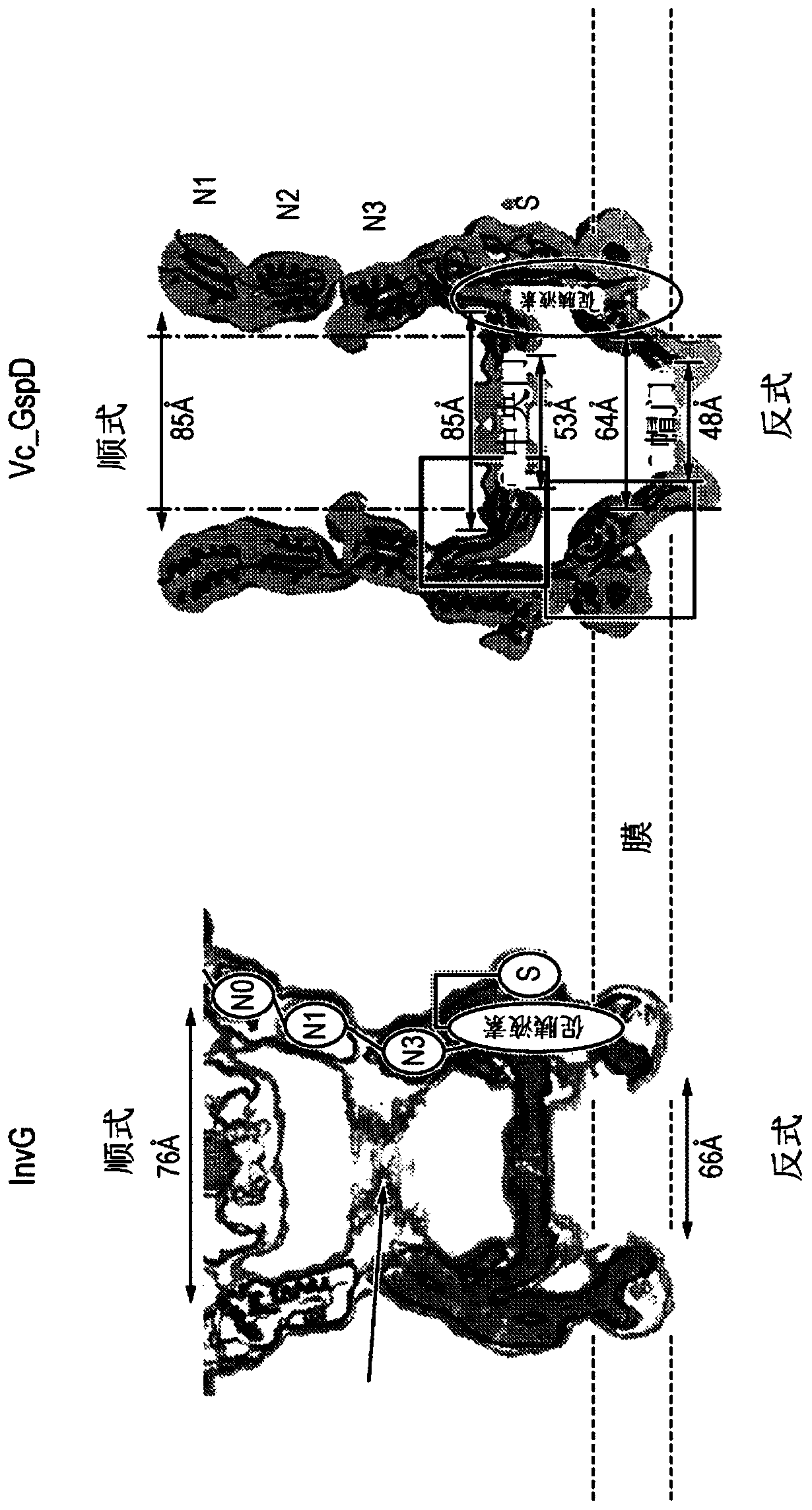
[0302] Design, expression and purification of embodiment 3 GspD mutants
[0303] Using the V. cholerae GspD sequence shown in SEQ ID NO: 32 as a starting sequence, GspD mutants were designed as shown in the table below.
[0304] Table 1: DNA capture mutants
[0305]
[0306]
[0307] Table 2: Increase the shrink size of the central door
[0308]
[0309] *Selected as background for further mutational design
[0310] Table 3: Stabilizing Central Doors
[0311]
[0312]
[0313] Table 4: Stabilizing Capgate: Removing Charge
[0314]
[0315] Table 5: Stabilizing Cap Doors
[0316]
[0317] Table 6: Shrinkage and cap extreme mutants
[0318]
[0319]
[0320] GspD mutants were expressed and purified in vitro using the NEB Pure Expression Kit. Set up the reaction as shown below.
[0321] Table 7: Reaction Mixture
[0322] components Volume (μL) Solution A 10 Solution B 7.5 35S methionine 1 rifampicin 0.8 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com