Synthesis method of Sb2S3 nanorods
A synthesis method and nanorod technology, applied in the direction of nanotechnology, chemical instruments and methods, antimony compounds, etc., can solve problems such as complicated procedures, long reaction time, and lack of S, and achieve simple procedures, simple procedures, and good product quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
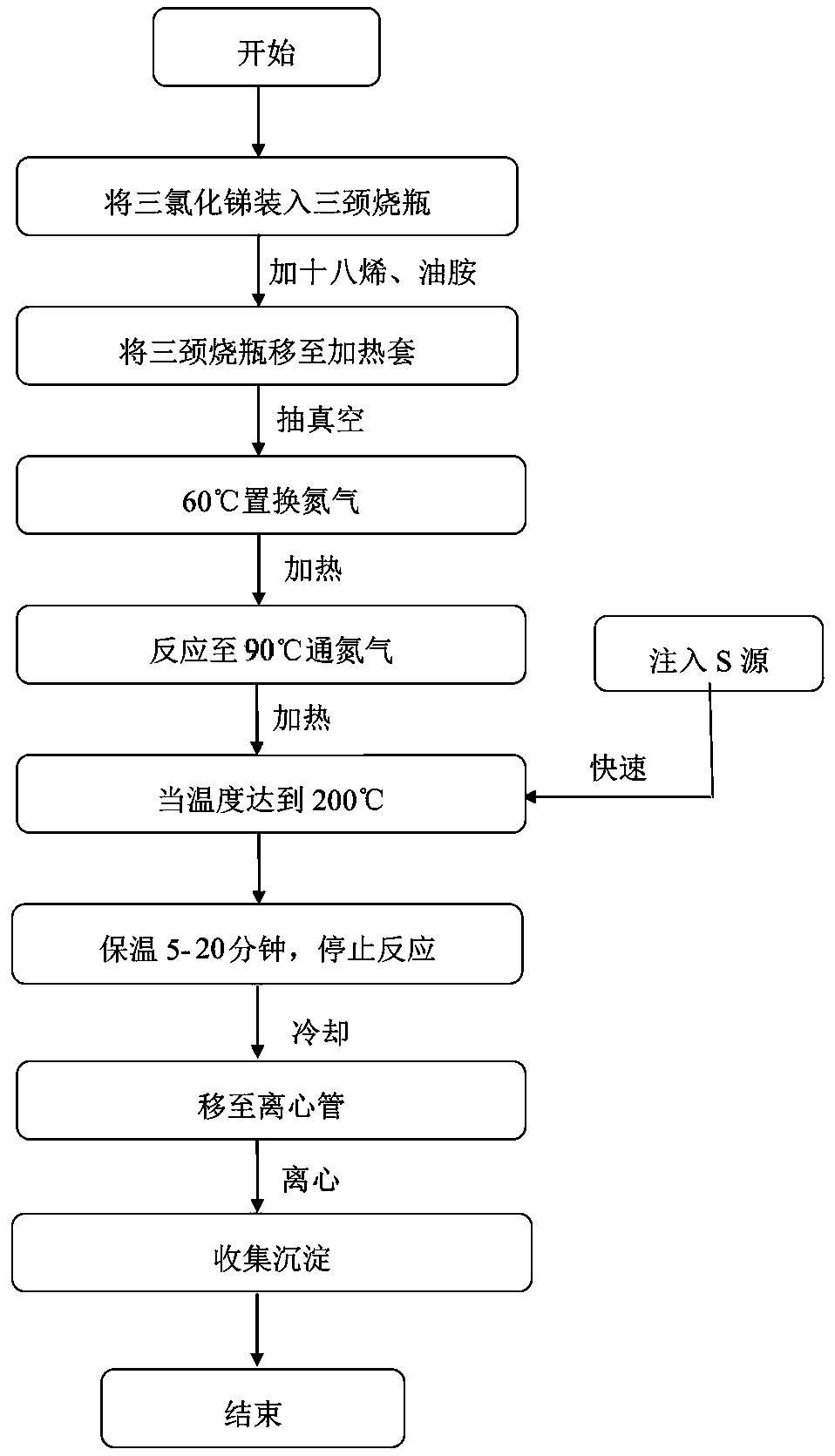
[0024] Such as figure 2 Shown, preparation process of the present invention is as follows:
[0025] (1) Preparation of sulfur source
[0026] Weigh 0.16g of high-purity sulfur (s, 99.99%) and place it in a three-necked flask, add 5mL of oleylamine (C 18 h 37 N, content 80-90%). Quickly connect the three-necked flask to the built device, turn on the vacuum pump to evacuate, turn on the heating mantle for heating and stirring (magnetic stirring), and when the reaction temperature reaches 60°C, open the nitrogen valve to fill the three-necked flask with nitrogen. Then evacuate again, and continue to heat up after replacing nitrogen three times like this. When the temperature reaches 90°C and the vacuum is pumped until there are no bubbles on the surface of the liquid, open the nitrogen valve and turn off the vacuum pump. Stop the reaction until all the sulfur in the three-necked flask is dissolved. When the temperature is cooled to 50°C, remove the three-necked flask, and ...
Embodiment 2
[0030] (1) Preparation of sulfur source
[0031] Weigh 0.16g of high-purity sulfur (s, 99.99%) into a three-necked flask, add 5mL of oleylamine (C 18 h 37 N, content 80-90%). Quickly connect the three-necked flask to the built device, turn on the vacuum pump to evacuate, turn on the heating mantle for heating and stirring (magnetic stirring), and when the reaction temperature reaches 60°C, open the nitrogen valve to fill the three-necked flask with nitrogen. Then evacuate again, and continue to heat up after replacing nitrogen three times like this. When the temperature reaches 90°C and the vacuum is pumped until there are no bubbles on the surface of the liquid, open the nitrogen valve and turn off the vacuum pump. Stop the reaction until all the sulfur in the three-necked flask is dissolved. When the temperature is cooled to 50°C, remove the three-necked flask, and put the prepared sulfur source into a vial for later use.
[0032] (2) Sb 2 S 3 preparation of
[0033]...
Embodiment 3
[0035] (1) Preparation of sulfur source
[0036] Weigh 0.16g of high-purity sulfur (s, 99.99%) into a three-necked flask, add 5mL of oleylamine (C 18 h 37 N, content 80-90%). Quickly connect the three-necked flask to the built device, turn on the vacuum pump to evacuate, turn on the heating mantle for heating and stirring (magnetic stirring), and when the reaction temperature reaches 60°C, open the nitrogen valve to fill the three-necked flask with nitrogen. Then evacuate again, and continue to heat up after replacing nitrogen three times like this. When the temperature reaches 90°C and the vacuum is pumped until there are no bubbles on the surface of the liquid, open the nitrogen valve and turn off the vacuum pump. Stop the reaction until all the sulfur in the three-necked flask is dissolved. When the temperature is cooled to 50°C, remove the three-necked flask, and put the prepared sulfur source into a vial for later use.
[0037] (2) Sb 2 S 3 preparation of
[0038]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com