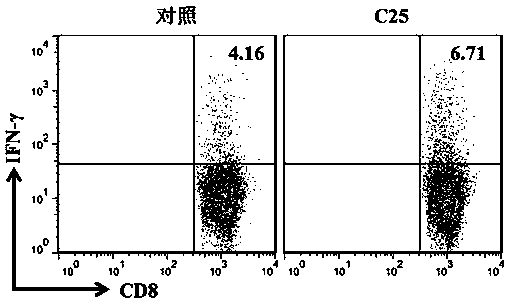
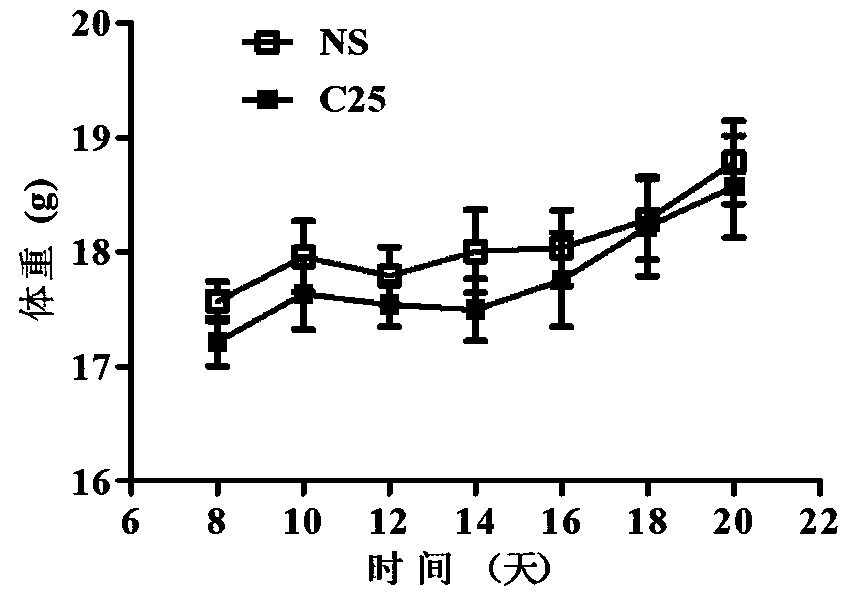
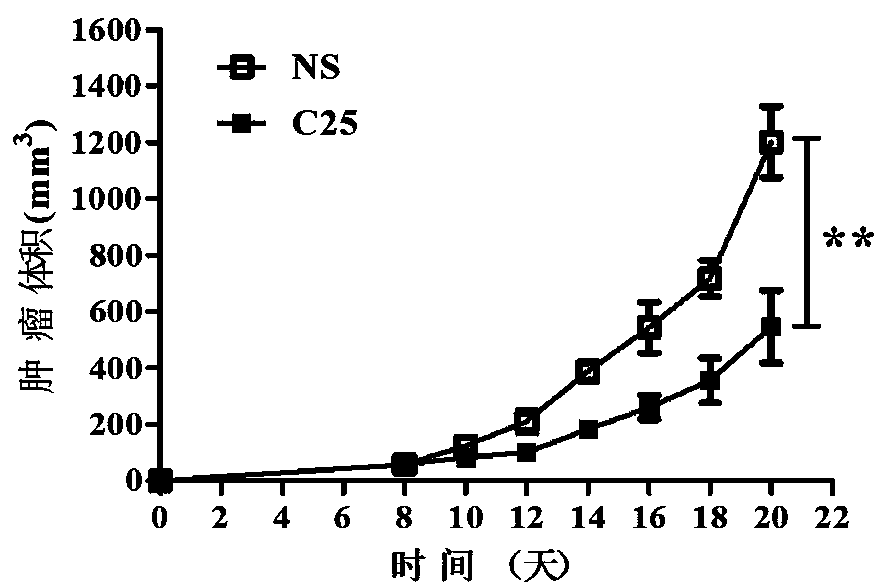
LAG-3 protein affinity cyclopeptide and application thereof
A LAG-3, cyclic peptide technology, applied in the field of affinity cyclic peptides, can solve the problems of hindering immune response TCR signal transduction, preventing binding, etc., and achieves good medical application prospects, good effects, and obvious effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Determination of phage titer
[0042] Inoculate a single colony of ER2738 in 10 mL LB medium, and culture it on a shaker until logarithmic phase (OD 600nm value around 0.5). Phage were serially diluted 10-fold with LB medium. Dilution range: Amplified phage culture supernatant: 10 8 ~10 12 ; Unamplified panning eluates: 10 1 ~10 5 . Put 200 μL of bacteria that have reached the logarithmic phase into a microcentrifuge tube, and then add 10 μL of different dilutions of phage to each tube, shake and mix quickly, and incubate at room temperature for 5 minutes. Add the infected bacteria to the upper agar culture tube pre-warmed at 45°C, mix quickly, and immediately pour it on the LB / IPTG / Xgal plate pre-warmed at 37°C to spread evenly. After the plate was cooled for 15 minutes, it was inverted and incubated overnight at 37°C. Check the plate the next day, the count is ~10 2 The number of spots on the plate of phage. This number was then multiplied by the dilutio...
Embodiment 2
[0078] (1) Human LAG-3 protein labeling: adjust the protein concentration to 2-20 μM with labeling buffer, take 100 μL for later use; add 30 μL 100% DMSO to the solid fluorescent dye, vortex to mix the dye; use the labeling buffer to adjust the concentration of the dye Adjust to 2-3 times the protein concentration; mix the protein and dye at a volume ratio of 1:1, and incubate at room temperature for 30 minutes in the dark.
[0079] (2) Purification of labeled human LAG-3 protein: Take out the top cover of the purification column, pour out the storage solution in it, take off the bottom cover and put it into a 15mL test tube, add 3mL of 10% Tween-20Tris-T buffer to balance and wash for purification Column, a total of 3 times; add the protein labeled in the previous step to the purification column, let the sample completely enter the column bed and discard the flow-through solution; put in a new 15mL test tube, add 600μL 10% Tween-20Tris-T buffer and collect Eluate; after testi...
Embodiment 3
[0083] (1) Replace THP-1 cells in good growth state with fresh DMEM medium (containing 0.3% FBS + 0.05mM β-mercaptoethanol) and place at 37°C, 5% CO 2 After culturing in the incubator for 4 hours, add 80ng / mL rhIFN-γ to stimulate THP-1 cells, mix well and place in the incubator for 48 hours;
[0084] (2) The blocking reaction was carried out in a 1.5mL EP tube, and the reaction system of 50μL PBS (pH7.2) buffer contained C25 cyclic peptide and 400ng of hLAG- 3-Fc protein, incubate at 4°C for 30 minutes;
[0085] (3) While incubating in step (2), collect THP-1 cells induced in step (1) and adjust the cell density to 5×10 5 Cells / sample, centrifuge at 3000rpm / min at 4°C for 5min to obtain the cells, wash once with 4°C pre-cooled PBS (pH7.2) buffer, centrifuge at 3000rpm / min at 4°C for 5min, discard the supernatant to obtain the cell pellet;
[0086] (4) Add the mixture obtained in step (2) after 30 minutes of reaction to the cell pellet obtained in step (3), vortex gently to m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com