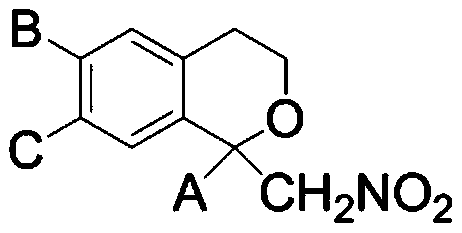
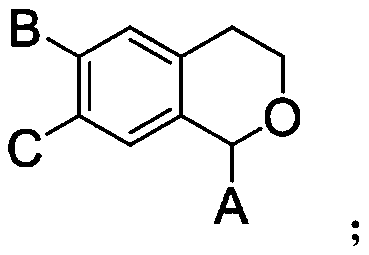
Preparation method of alpha, alpha-di-substituted iso-chroman compound
An isochroman and compound technology, which is applied in the field of preparation of α,α-disubstituted isochroman compounds, can solve problems such as sensitivity, and achieve the effects of simple operation, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Taking the preparation of the compound 1-nitromethyl-1-phenylisochroman as an example, the raw materials used and the preparation method are as follows:
[0037]
[0038] Mix 161mg (0.6mmol) of 1-phenylisochromatium (0.6mmol) and 238mg (0.72mmol) of triphenylmethyltetrafluoroborate in dichloroethane at 20°C, stir and react for 1 hour, add 160uL (3.0mmol) ) Nitromethane, react at 80°C for 24 hours. When the reaction is over, add 2 mL of distilled water to the system, extract three times with 10 mL of dichloromethane, combine the organic phases, dry with anhydrous sodium sulfate, filter with suction, concentrate, and give the crude product Column chromatography separation (dichloromethane and n-hexane) was performed to obtain 1-nitromethyl-1-phenyl isochroman as a white solid with a yield of 95%.
[0039] The NMR characterization data are: 1 H NMR(600MHz, CDCl 3 )δ7.40–7.39(m,2H),7.36–7.26(m,7H),5.06(d,J=12.1Hz,1H), 4.88(d,J=12.1Hz,1H),4.05(ddd,J = 11.6, 6.0, 1.8 Hz, 1H), 3.6...
Embodiment 2
[0041] Taking the preparation of 1-nitromethyl-1-p-tert-butylphenyl isochroman as an example, the raw materials used and the preparation method are as follows:
[0042]
[0043] In Example 2, the 1-phenyl isochroman used in Example 1 was replaced with an equimolar 1-tert-butyl phenyl isochroman. The other steps were the same as in Example 1, and a white solid was prepared. The yield was 92%.
[0044] The NMR characterization data are: 1 H NMR(600MHz, CDCl 3 )δ8.20(d,J=8.8Hz,2H), 7.65(d,J=8.8Hz,2H), 7.36–7.26(m,4H), 5.10(d,J=12.1Hz,1H), 4.84( d, J = 12.1Hz, 1H), 4.03 (ddd, J = 11.6, 6.0, 1.8 Hz, 1H), 3.69 (td, J = 11.4, 3.7 Hz, 1H), 3.06–3.01 (m, 1H), 2.64 –2.60(m,1H),1.37(s,9H); 13 C NMR(151MHz, CDCl 3 )δ145.5,139.8,139.0,132.4,128.5,128.4,126.9,125.7,123.7,122.8,82.0,79.2,61.1,35.2,31.1.HRMS(ESI)m / z:C 20 H 23 NNaO 3 [M+Na] + The theoretical value is 348.1576, and the measured value is 348.1558.
Embodiment 3
[0046] Taking the preparation of the compound 1-nitromethyl-1-p-chlorophenyl isochroman as an example, the raw materials used and the preparation method are as follows:
[0047]
[0048] In Example 3, the 1-phenyl isochroman used in Example 1 was replaced with an equimolar 1-chlorophenyl isochroman. The other steps were the same as in Example 1, and the white solid was prepared. The yield Is 92%.
[0049] The NMR characterization data are: 1 H NMR(600MHz, CDCl 3 )δ7.36–7.29(m,4H),7.26–7.22(m,4H),5.07(d,J=12.1Hz,1H), 4.93(d,J=12.1Hz,1H),4.05–3.98(m ,1H), 3.71–3.67(m,1H),3.06–3.01(m,1H), 2.64–2.60(m,1H); 13 C NMR(151MHz, CDCl 3 )δ139.8,138.6,133.8,132.4,128.4,128.0,127.7,126.9,125.7,122.8,81.7,78.4,59.6,26.9.HRMS(ESI)m / z:C 16 H 14 ClNNaO 3 [M+Na] + The theoretical value is 326.0560, and the measured value is 326.0579.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com