A method for preparing battery-grade iron phosphate from lithium phosphate waste
A lithium phosphate, iron phosphate technology, applied in chemical instruments and methods, lithium carbonate;/acid carbonate, phosphorus compounds, etc., can solve the problems of low mother liquor concentration, hydrogen chloride gas overflow, poor operating conditions, etc. Achieve the effect of good operating environment, high process controllability and no waste residue discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
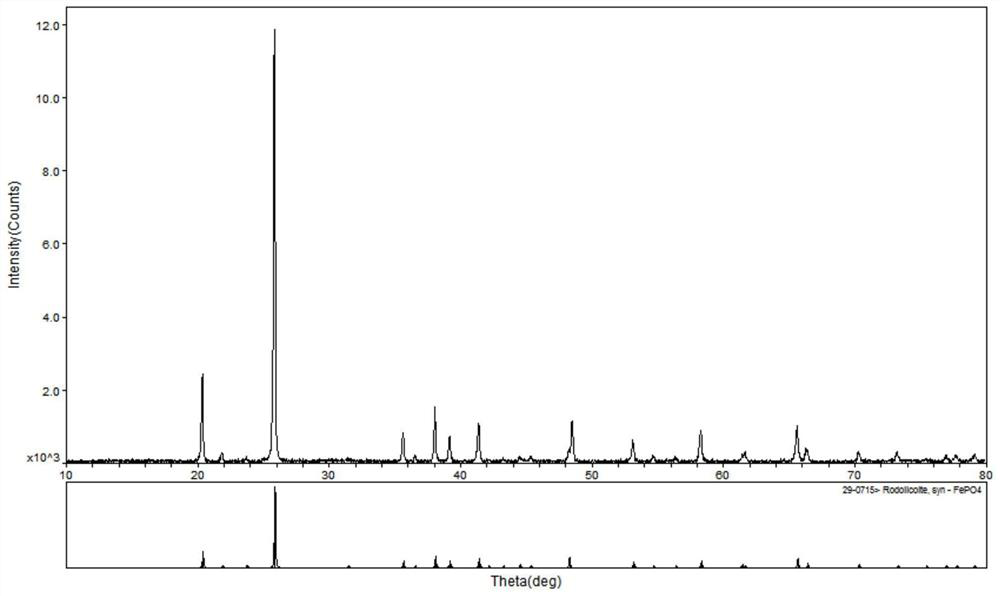
Image
Examples
Embodiment 1
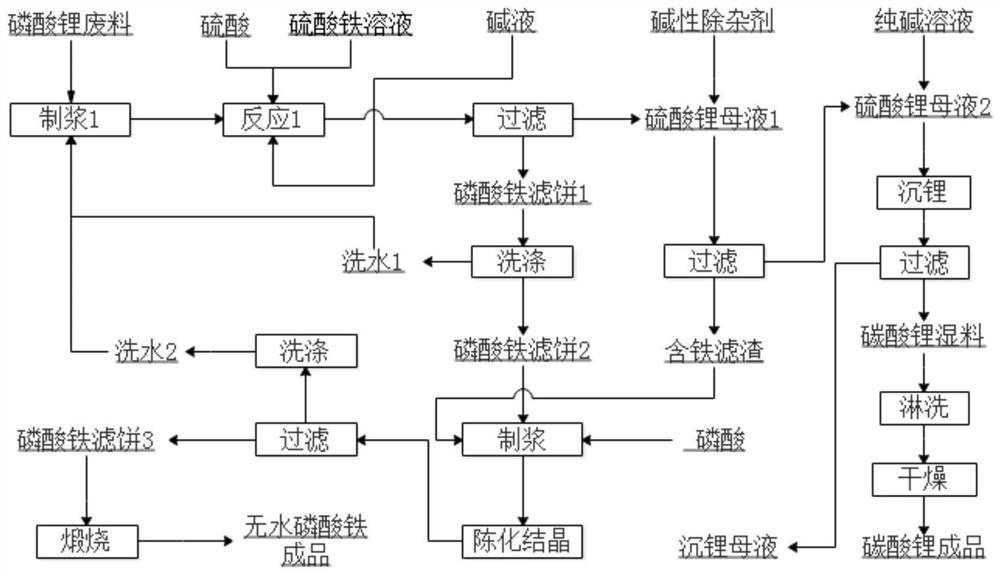
[0043] Embodiment 1 of the present invention is: a method for preparing battery-grade iron phosphate from lithium phosphate waste, the operation process of which is as follows figure 1 As shown, it specifically includes the following steps:
[0044](1) Collect the lithium phosphate waste produced in the process of producing lithium compounds in a certain factory, and detect the components in the waste. The results are shown in Table 1 below:
[0045] Table 1 Lithium phosphate waste composition analysis table
[0046]
[0047] (2) Weigh 5000g of the above-mentioned lithium phosphate waste material and add it to 5L of water (control the mass ratio of lithium phosphate waste material to pure water or washing water at 1:(1-3) so as to increase the concentration of soluble lithium salt and increase The particle size of amorphous ferric phosphate is better for filtering and washing. The present invention adopts the most preferred ratio of 1:1), and after stirring evenly, add 382...
Embodiment 2
[0052] Embodiment 2 of the present invention is: a method for preparing battery-grade iron phosphate by using lithium phosphate waste, its operation process is as follows figure 1 As shown, it specifically includes the following steps:
[0053] (1) Weigh 10 kg of lithium phosphate waste from the same batch of the above-mentioned examples, add it to 30 L of water, add 9150 g of sulfuric acid (wt% = 98%) after stirring evenly, and after the lithium phosphate is completely dissolved, add 23.5 kg of hexahydrate evenly within 60 minutes Ferric chloride (wt%=98%), the speed of reaction solution is 600r / min, adding potassium hydroxide solution to adjust the pH value of the solution to 3.0, after 80°C reaction time of 60min, filter to obtain soluble lithium salt solution I and amorphous Iron Phosphate Precipitation II.
[0054] (2) Add the alkaline impurity removal agent ammonia into the soluble lithium salt solution I, control the pH value of the solution to 4.5, and filter to obtai...
Embodiment 3
[0057] Embodiment 3 of the present invention is: a method for preparing battery-grade iron phosphate by using lithium phosphate waste, the operation process of which is as follows figure 1 shown, including the following steps:
[0058] (1) take by weighing 50kg lithium phosphate waste material, add in 100L water, add 11450g sulfuric acid (wt%=98%) after stirring evenly, after treating that lithium phosphate dissolves completely, evenly add 76kg ferric sulfate (wt%=99.5%) in 100min, The rotational speed of the reaction solution was 300r / min, the pH value of the solution was adjusted to 2.2 by adding ammonia water, and after a reaction time of 80 minutes at 60°C, the soluble lithium salt solution I and the amorphous iron phosphate precipitate II were obtained by filtration.
[0059] (2) Add alkaline impurity remover sodium carbonate solution into soluble lithium salt solution I, control the pH value of the solution to 4.0, and filter to obtain refined soluble lithium salt soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com