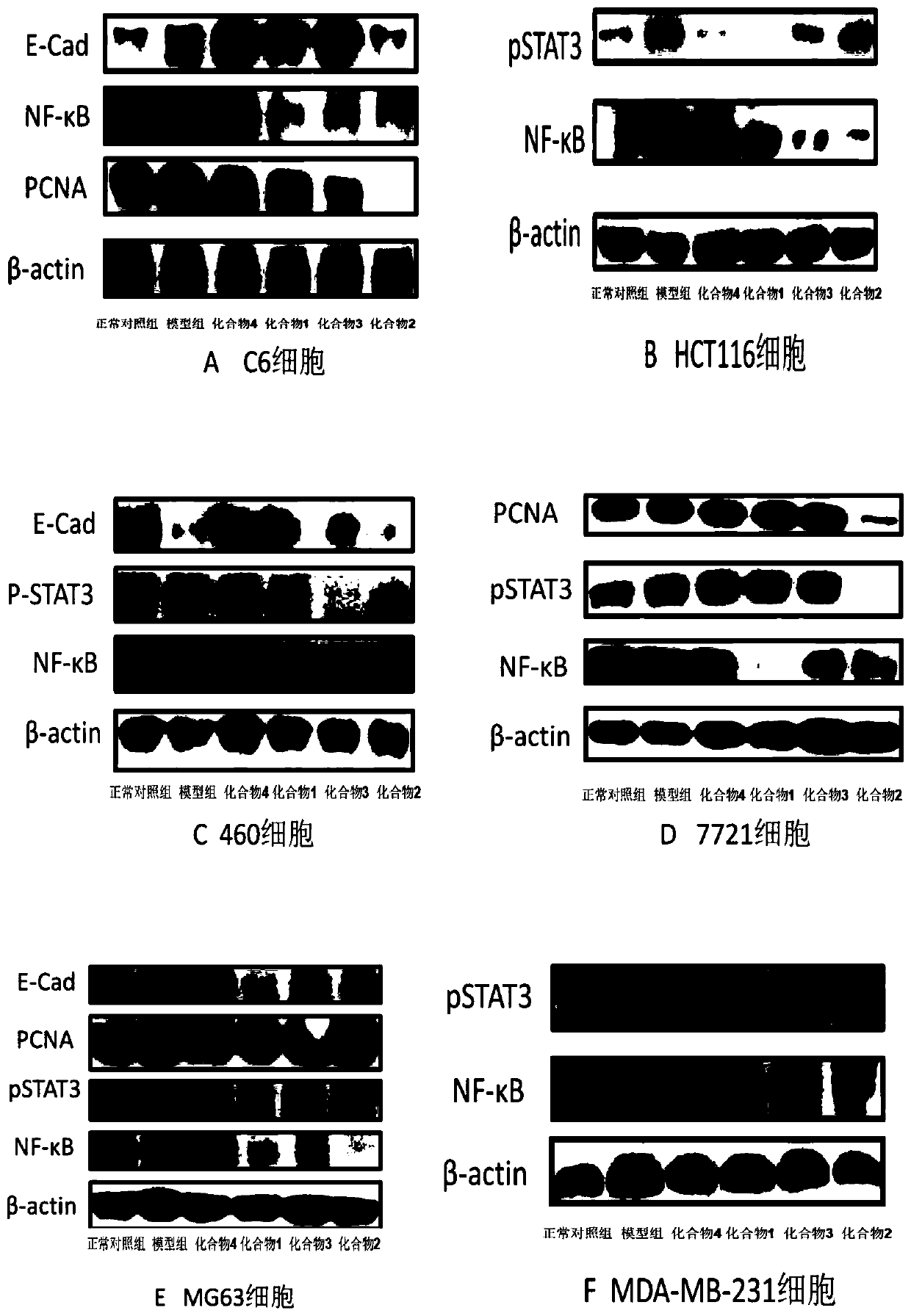
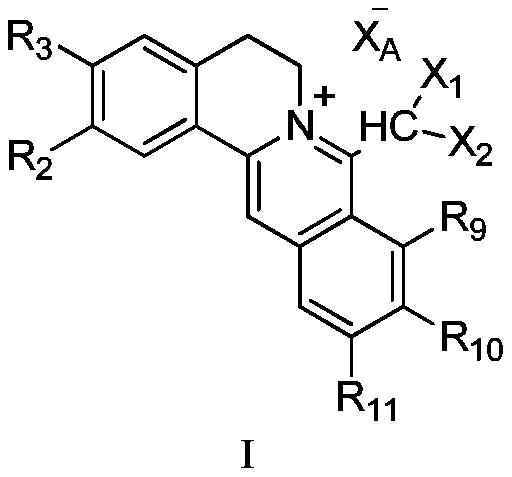
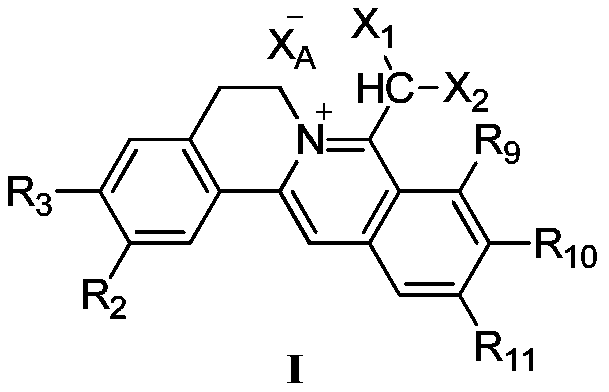
Hydrophilic berberine type derivative connected with R10 and R11 and application of hydrophilic berberine type derivative to preparation of drugs
A technology of R11 and dihalomethyl berberine, which is applied in the field of 8-dihalomethyl berberine type quaternary ammonium salt compounds, can solve the problems of poor solubility and toxicity, the stability of the pharmacological action, the strength of the chemical structure, and the absence of Significantly improve druggability and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0101] The preparation process and structural identification data of the active compounds of the present invention, wherein the compound number corresponds to the specific compound number in the content of the present invention.
[0102] One, the preparation experiment example of compound of the present invention
experiment example (1
[0103] Experimental example (1) Preparation process and structure identification data of compound 2
[0104] Dissolve isotropine hydrochloride (1.0g, 2.81mmol) in 80ml of chloroform in a reaction flask, add NaH (450mg, 18.75mmol) under ice, slowly warm up to room temperature and stir for 24h, then concentrate the reaction solution under reduced pressure to Obtain a residue; first add ethyl acetate to the residue, then add an appropriate amount of pure water, extract 3 times with ethyl acetate, combine the ethyl acetate extracts, and use anhydrous MgSO 4 Dry, filter, and evaporate the filtrate to dryness. The resulting crude product is purified by silica gel column chromatography, eluting with petroleum ether / ethyl acetate (3 / 1, v / v), and the eluted part is concentrated to obtain 8-trichloromethyldihydro 995 mg of isotropine yellow solid, yield 80.7%. 1 HNMR (500MHz, CDCl 3 )δ: 2.68 (d, J = 15.5 Hz, 1H, NCH 2 C H 2 ),3.35-3.40(m,1H,NCH 2 C H 2 ), 3.70(t, J=8.5Hz, 1H, NC...
experiment example (2
[0105] Experimental example (2) Preparation process and structure identification data of compound 4
[0106] Take palmatine hydrochloride (5.0g, 12.9mmol) and dissolve in 200ml chloroform in the reaction flask, further add concentrated ammonia water 30ml; Stir and react at room temperature after 24h, separate the chloroform layer, wash the chloroform solution with water for 2 times, with anhydrous MgSO 4 Dry and filter; the crude product after the filtrate was evaporated to dryness was purified by silica gel column chromatography, eluted with dichloromethane, and the eluate was evaporated to dryness to give 4.545 g of 8-trichloromethyldihydropalmatine as a pale yellow solid, the yield 74.9%. 1 H NMR (500MHz, CDCl 3 )δ: 2.76 (d, J=15.5Hz, 1H, NCH 2 C H 2 ),3.42(m,1H,NCH 2 C H 2 ), 3.77(m,1H,NC H 2 CH 2 ), 3.88 (br ov, 4H, NC H2 CH 2 ,ArOCH 3 ),3.90(s,3H, ArOCH 3 ),3.948(s,3H,ArOCH 3 ),3.953(s,3H,ArOCH 3 ),5.66(s,1H, CH-CCl 3 ),6.12(s,1H,C=CH),6.65(s,1H,ArH),6....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com