r 9 with r 10 Linked hydrophilic berberine derivatives and their application in the preparation of medicines
A dihalomethyl berberine and drug technology, applied in the field of 8-dihalomethyl berberine type quaternary ammonium salt compounds, can solve the problem of no significant improvement in drugability and toxicity, pharmacological action strength, chemical structure stability , poor solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
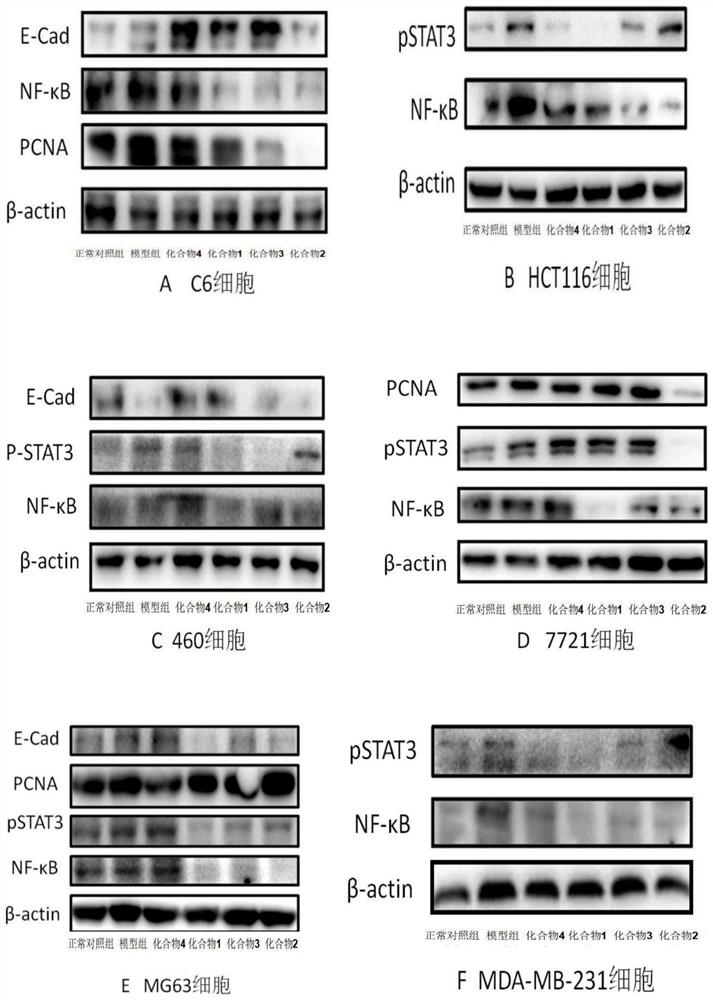
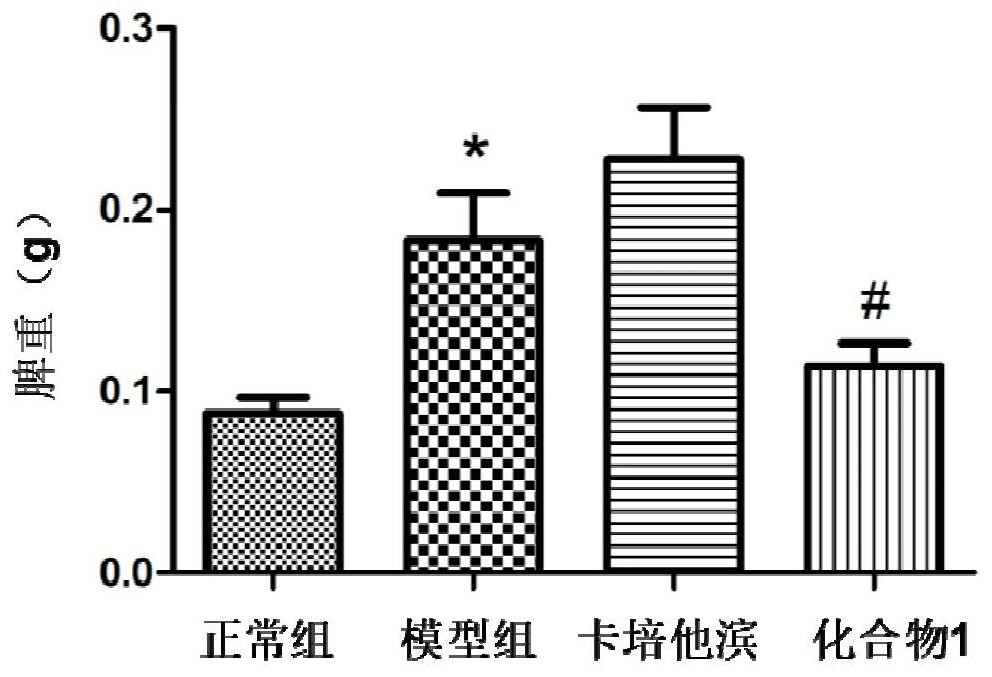
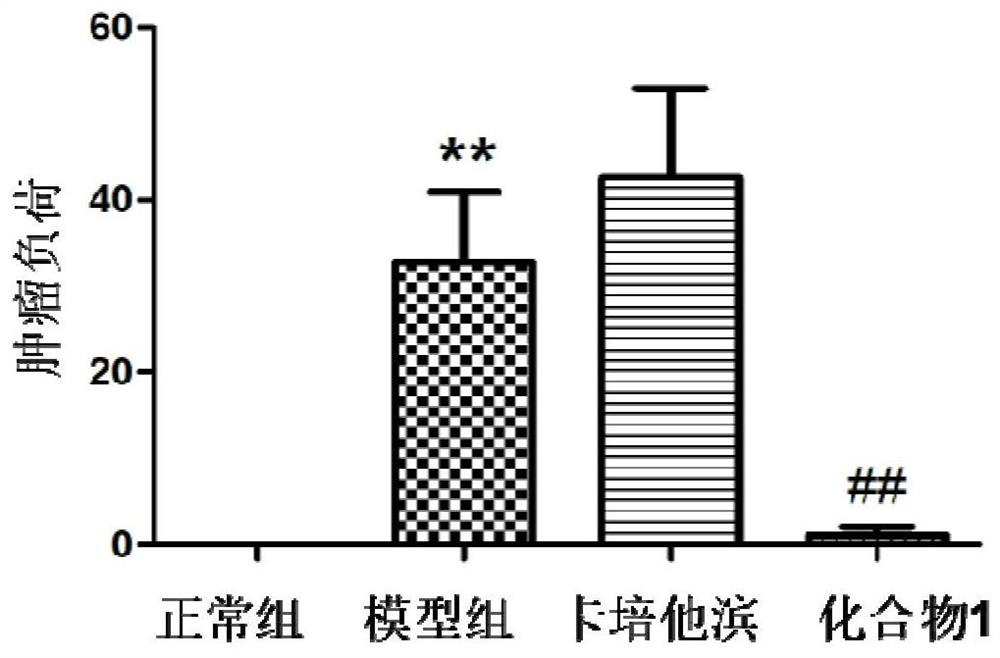
[0106] The preparation process and structural identification data of the active compounds of the present invention, wherein the compound number corresponds to the specific compound number in the content of the present invention.
[0107] One, the preparation experiment example of compound of the present invention
experiment example (1
[0108] Experimental example (1) Preparation process and structure identification data of compound 1
[0109] Coptisine hydrochloride (2.0g, 5.62mmol) was dissolved in 200mL chloroform-methanol (3:1) mixed solvent in the reaction flask, added concentrated ammonia water 24ml, stirred and reacted at room temperature for 24h, then separated the chloroform layer, and The chloroform layer was washed twice with water and washed with anhydrous MgSO 4 After drying, it was filtered; the filtrate was evaporated to dryness, and the resulting crude product was purified by silica gel column chromatography, eluting with dichloromethane, and the eluate was concentrated to obtain 617 mg of 8-trichloromethyldihydrocoptisine as a light yellow solid, with a yield of 25.0%. 1 H NMR (500MHz, CDCl 3 )δ:2.63–2.78(m,1H,NCH 2 C H 2 ),3.35(m,1H, NCH 2 C H 2 ),3.71(m,1H,NC H 2 CH 2 ),3.78-3.90(m,1H,NC H 2 CH 2 ),5.42 (s,1H,CH-CCl 3 ),5.91(br,1H,OCH 2 O),5.95(br,2H,OCH 2 O),6.02(br,2H,OCH ...
experiment example (2
[0110] Experimental example (2) Preparation process and structure identification data of compound 3
[0111] Dissolve berberine hydrochloride (2.0g, 5.38mmol) in 60ml chloroform in a reaction flask, add 24ml of concentrated ammonia water, stir and react at room temperature for 24h, then separate the chloroform layer, wash the chloroform solution with water twice, and wash with Anhydrous MgSO 4 After drying the chloroform solution, filter it, and evaporate the filtrate to dryness to obtain a residue; the residue is separated and purified by silica gel column chromatography, eluted with dichloromethane, and the eluent is evaporated to dryness to obtain 8-trichloromethyldihydroberberis The base is 1.775 g of light yellow solid, the yield is 78.9%. 1 H NMR (500MHz, CDCl 3 )δ: 2.73 (d, J=15.5Hz, 1H, NCH 2 C H 2 ),3.33(m, 1H,NCH 2 C H 2 ),3.71(m,1H,NC H 2 CH 2 ),3.87(ov,4H,ArOCH 3 , NC H 2 CH 2 ),3.94(s,3H,ArOCH 3 ),5.65(s,1H,CH-CCl 3 ),5.94(s,2H,OCH 2 O),6.08(br s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com