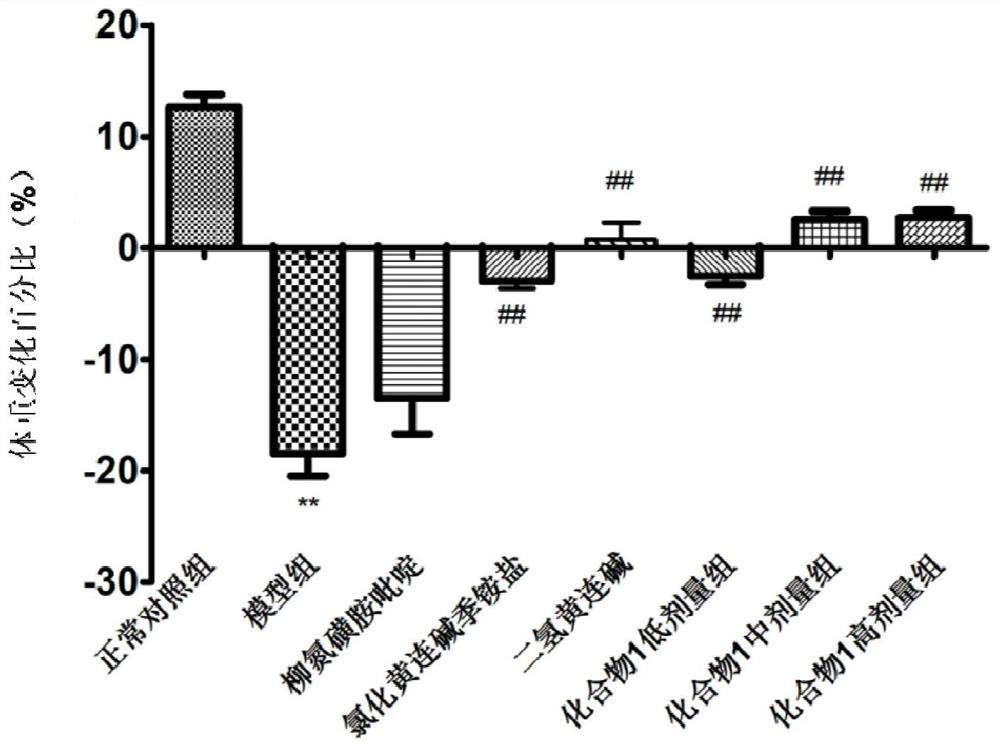
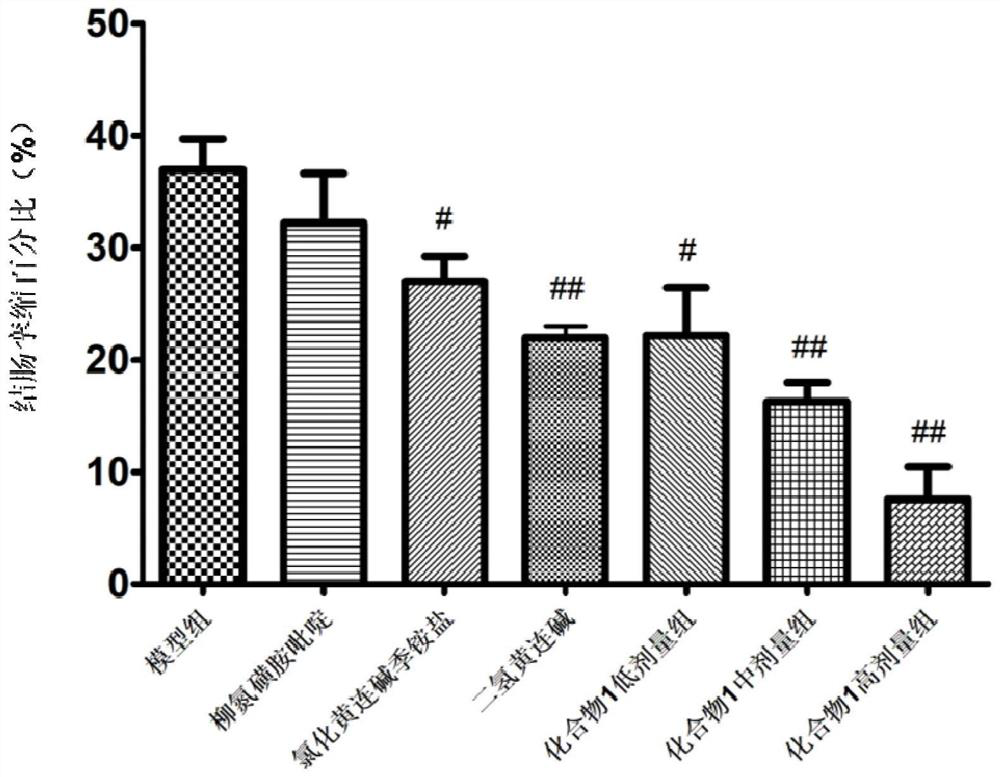
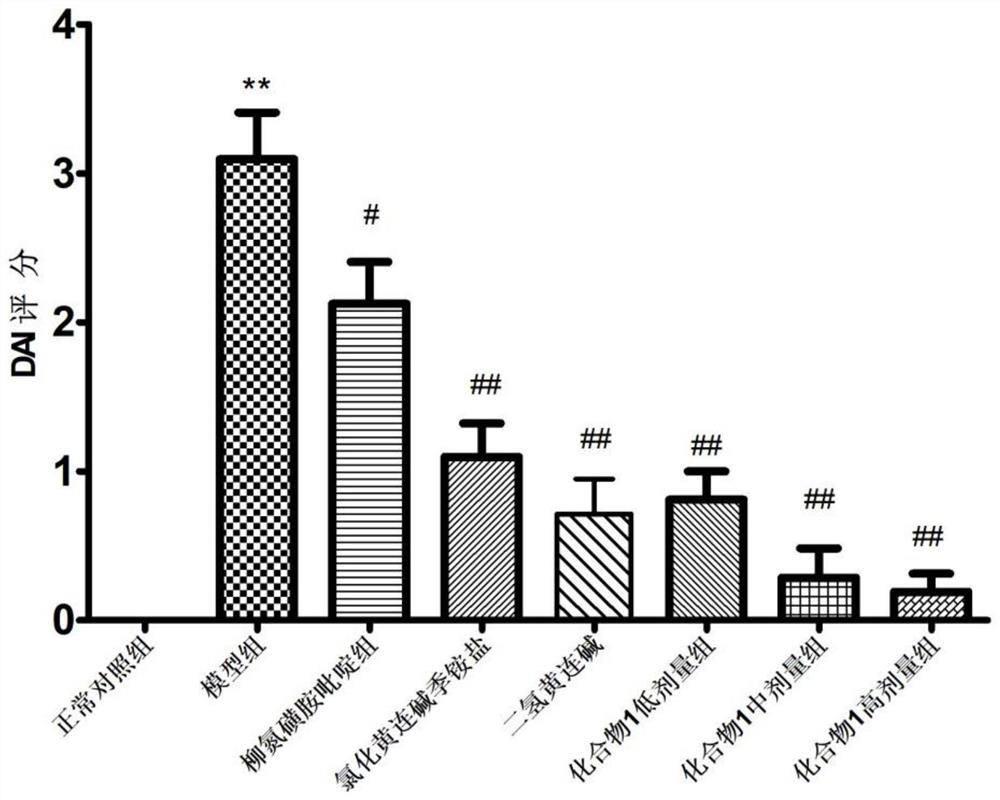
Salicylic acid berberine type alkaloid quaternary ammonium salt and application in preparation of medicines
A technology of quaternary ammonium salt compound and berberine, applied in the field of medicine, can solve problems such as limited clinical benefit, and achieve the effects of remarkable safety, remarkable practical value and excellent solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0060] The preparation process and structural identification data of the active compounds of the present invention, wherein the compound number corresponds to the specific compound number in the content of the present invention.
[0061] 1. Experimental example of compound preparation of the present invention
experiment example (1
[0062] Experimental example (1): Synthesis and structure identification data of compound 1 of the present invention
[0063] Take by weighing berberine chloride quaternary ammonium salt (2.00g, 5.62mmol) in the reaction flask, add 5N aqueous sodium hydroxide solution (12ml), then add acetone (4ml, 54.27mmol) dropwise, stir at room temperature for 4h, the raw material reaction completely. The reaction mixture was suction-filtered, and the filter cake was washed with water until it became neutral to obtain 1.99 g of 8-acetonyl dihydrocopterine as a light yellow solid, with a yield of 93.87%.
[0064] Weigh 5-aminosalicylic acid (43mg, 0.28mmol) in a reaction flask, add DMSO (3ml), sonicate until completely dissolved, then add 8-acetonyldihydrocoptisine (100mg, 0.26mmol) under stirring at room temperature, After the addition, the reaction was carried out at 25°C for 5.5h until the raw materials were completely reacted; adding tetrahydrofuran (6ml) to the reaction mixture to dilu...
experiment example (2
[0065] Experimental example (2): Synthesis and structural identification data of compound 2 of the present invention
[0066] Weigh the quaternary ammonium salt of berberine chloride (3.84g, 10.80mmol) in a reaction flask, add 5N aqueous sodium hydroxide solution (100ml), then add acetone (10ml, 0.14mol) dropwise, and heat and stir at 50°C for 4h , the raw material reacted completely. The reaction mixture was suction filtered, and the filter cake was washed with water until neutral. Obtain the light yellow solid crude product, then recrystallize with a mixed solvent of acetone and water (acetone:water=3:1, v / v) to obtain 2.26g of yellow granular crystals of 8-acetonyl dihydroisocortisine, yield 55.52%.
[0067] Weigh 5-aminosalicylic acid (43mg, 0.28mmol) in a reaction flask, add DMSO (3ml), sonicate until completely dissolved, then add 8-acetonyl dihydroisoberisine (100mg, 0.26mmol) under stirring at room temperature After the addition, the reaction was carried out at 25°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com