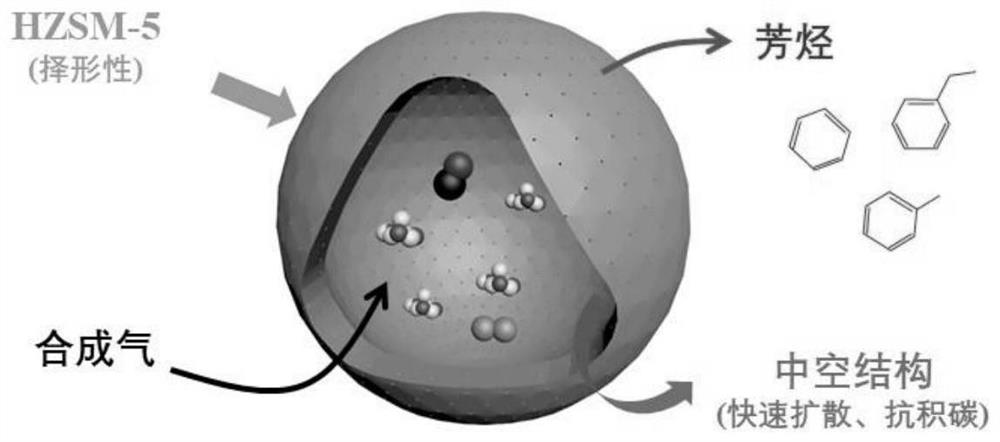
A composite catalyst for directly producing aromatics from synthesis gas and its preparation method
A composite catalyst and synthesis gas technology, which is applied in the preparation of liquid hydrocarbon mixtures, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of no stability research, catalyst deactivation, etc., and achieve good industrial application prospects and high aromatics selection performance, excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
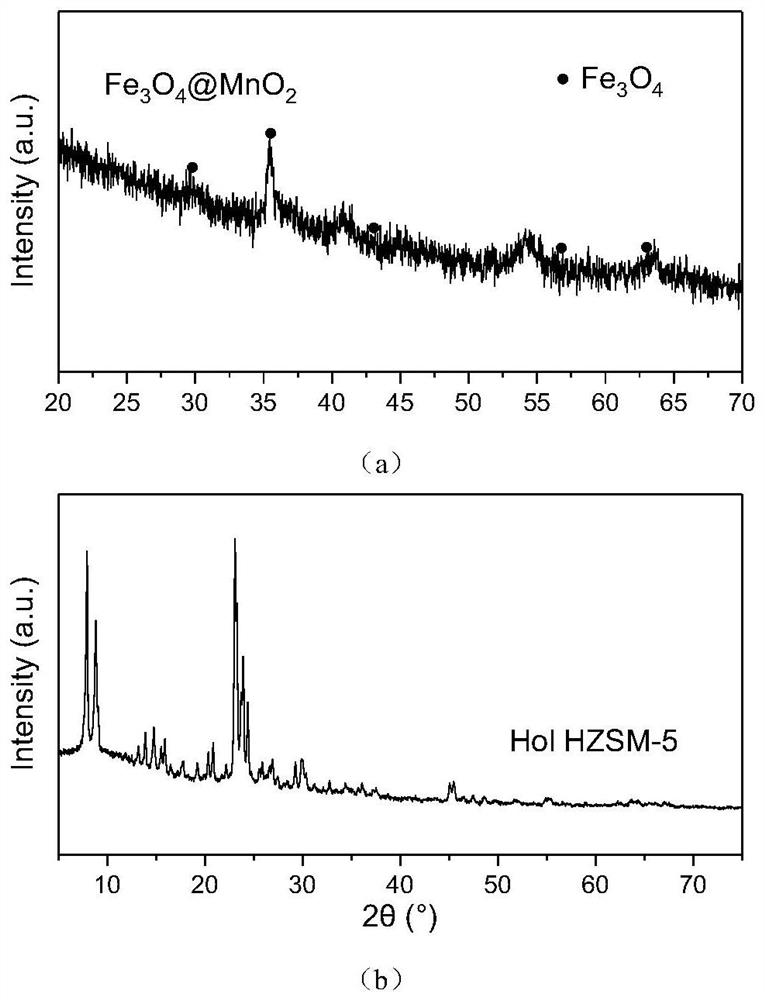
[0040] Preparation of Fe with Fe:Mn molar ratio of 1:1 3 o 4 @MnO 2 catalyst:
[0041] Dissolve 100mmol of ferrous sulfate in 1L of deionized water, then add 100g of PVP, and stir until completely dissolved. The above solution was aged in an oil bath at 70°C for 10 h, and then 400 mmol of sodium hydroxide and 100 mmol of potassium permanganate were added. The precipitate was washed with deionized water and dried at 100°C to obtain Fe with a molar ratio of Fe:Mn of 1:1. 3 o 4 @MnO 2 .
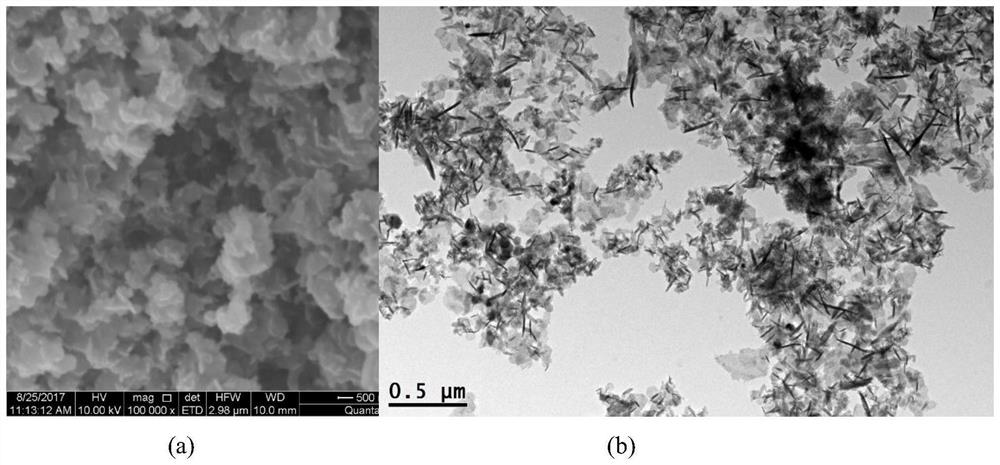
[0042] Preparation of hollow HZSM-5 zeolite:
[0043] Mix HZSM-5 zeolite with 1.0M tetrapropylammonium hydroxide (TPAOH) aqueous solution at a solid-to-liquid ratio of 1g / 50ml. Hydrothermal reaction at 150°C for 120h. After centrifugal separation, the solid product was washed with deionized water, dried and then calcined at 550°C for 10 hours to obtain a hollow HZSM-5 zeolite.
[0044] Preparation of composite catalyst:
[0045] Will Fe 3 o 4 @MnO 2 Physically mix with hollow HZSM-...
Embodiment 2
[0054] Preparation of Fe with Fe:Mn molar ratio of 2:1 3 o 4 @MnO 2 catalyst:
[0055] Dissolve 100mmol of ferrous sulfate in 1L of deionized water, then add 100g of PVP, and stir until completely dissolved. The above solution was aged in an oil bath at 50° C. for 10 h, and then 500 mmol of sodium hydroxide and 50 mmol of potassium permanganate were added. The precipitate was washed with deionized water and dried at 100°C to obtain Fe with a molar ratio of Fe:Mn of 2:1. 3 o 4 @MnO 2 .
[0056] Preparation of hollow HZSM-5 zeolite:
[0057] Mix HZSM-5 zeolite with 0.8M tetrapropylammonium hydroxide (TPAOH) aqueous solution at a solid-to-liquid ratio of 1g / 5ml; hydrothermally react at 140°C for 5h; centrifuge, wash the solid product with deionized water, dry it at 400 Calcined at ℃ for 10h to obtain hollow HZSM-5 zeolite.
[0058] Preparation of composite catalyst:
[0059] Will Fe 3 o 4 @MnO 2 Physically mix with hollow HZSM-5 zeolite at a mass ratio of 1:5.
[00...
Embodiment 3
[0062] Preparation of Fe with Fe:Mn molar ratio of 4.5:1 3 o 4 @MnO 2 catalyst:
[0063] Dissolve 100mmol of ferrous sulfate in 1L of deionized water, then add 100g of PVP, and stir until completely dissolved. The above solution was aged in an oil bath at 30° C. for 10 h, and then 600 mmol of sodium hydroxide and 22.2 mmol of potassium permanganate were added. The precipitate was washed with deionized water and dried at 100°C to obtain Fe with a molar ratio of Fe:Mn of 4.5:1. 3 o 4 @MnO 2 .
[0064] Preparation of hollow HZSM-5 zeolite:
[0065] Mix HZSM-5 zeolite with 0.1M tetrapropylammonium hydroxide (TPAOH) aqueous solution according to the solid-to-liquid ratio of 1g / 30ml; hydrothermally react at 140°C for 48h; centrifuge, wash the solid product with deionized water, dry it at 450 Calcined at ℃ for 4h to obtain hollow HZSM-5 zeolite.
[0066] Preparation of composite catalyst:
[0067] Will Fe 3 o 4 @MnO 2 Physically mix with hollow HZSM-5 zeolite at a mass r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com