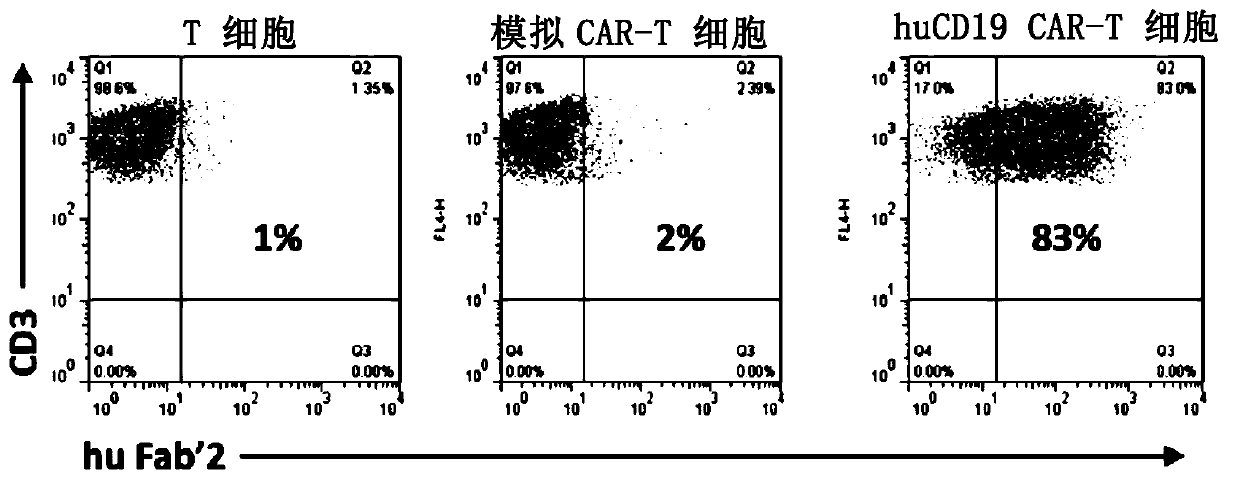
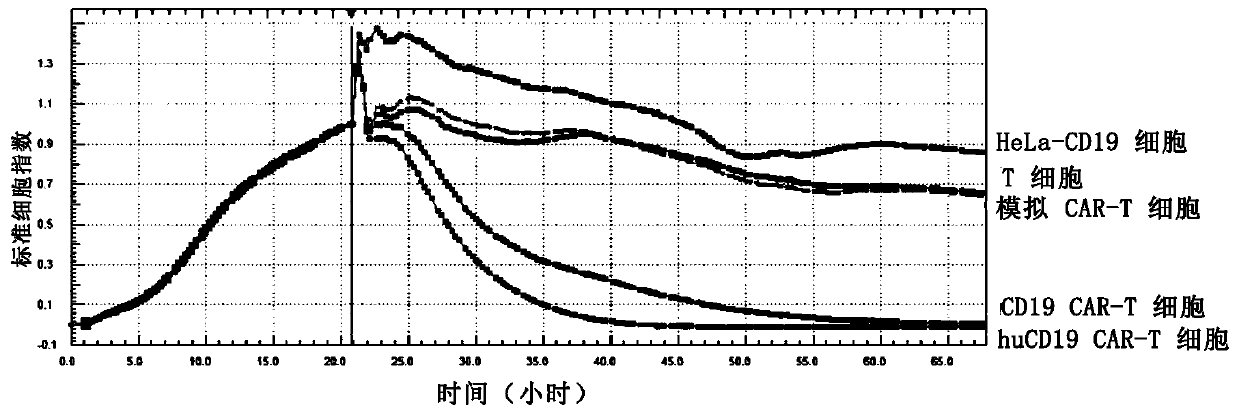
Humanized CD19 antigen binding single chain antibody, chimeric antigen receptor thereof, immune cell and application
A single-chain antibody, humanized technology, applied in blood/immune system cells, receptors/cell surface antigens/cell surface determinants, anti-receptors/cell surface antigens/cell surface determinants immunoglobulins, etc. It can solve the problems affecting the long-term curative effect and recurrence of CAR-T
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0150] In the present invention, a method for preparing CAR-immune cells is also provided, including:
[0151] (A) providing an immune cell to be modified; and
[0152] (B) introducing a CAR expression cassette into the immune cell to be engineered, wherein the CAR expression cassette expresses the CAR construct of the present invention, thereby obtaining an engineered immune cell.
[0153]Preferably, the method comprises the following steps: synthesizing the coding sequence of a humanized anti-human CD19 antigen chimeric antigen receptor (CAR); transfecting the obtained CAR coding sequence into a virus preparation cell through a vector system, and packaging it into a The CAR virus, and then transduce the CAR sequence to the immune cells through the virus or directly transduce the CAR sequence to the immune cells through electroporation and other methods.
[0154] In the present invention, there is no special limitation on the transfection method, and the transfection method ...
Embodiment 1
[0169] Design of humanized scFv against human CD19 antigen
[0170] Using the mouse anti-human CD19 antigen FMC63 antibody as the parent antibody, on the basis of the amino acid sequence of its scFv chain, the framework region was humanized and modified to design a humanized antibody against human CD19 antigen.
[0171] Firstly, the core sequence of FMC63 was determined by molecular docking simulation, and the amino acid sequences of the CDR regions of the heavy chain and light chain variable regions of the scFv sequence were determined, that is, CDR1, CDR2, and CDR3 of the light chain VL and CDR1, CDR2, and CDR3 of the heavy chain VH ; Then, in the NCBI / lgBLAST software, the human sequence in the database is analyzed and compared with the scFv sequence of FMC63, and the framework region sequence corresponding to the human antibody variable region sequence with higher homology is selected as the human template . The framework sequence of FMC63 was modified and replaced with t...
Embodiment 2
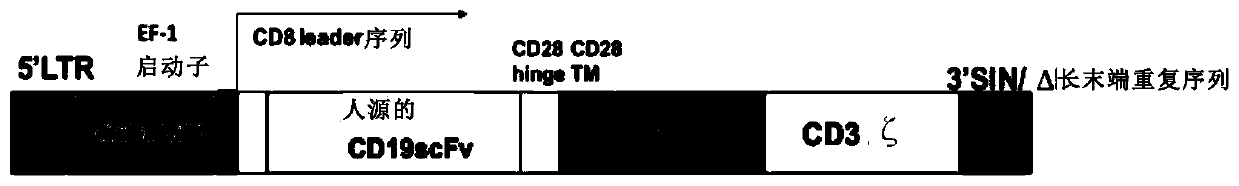
[0211] The nucleotide sequence of the chimeric antigen receptor (CAR) artificially synthesized against human CD19 is shown in SEQ ID No: 21, and EcoRI and XbaI restriction sites are added at both ends:
[0212]
[0213]
[0214] Use restriction endonucleases EcoRI and XbaI to carry out double enzyme digestion reactions on the lentiviral vector and the synthesized DNA sequence respectively. After the reaction is completed, use T4 DNA ligase (purchased from TaKaRa) to ligate the digested lentiviral vector and DNA fragments In response, a lentiviral vector expressing the chimeric antigen receptor was obtained. The preparation method of the lentiviral vector plasmid expressing the chimeric antigen receptor (CAR) refers to the instructions of the QIAGEN endotoxin-free plasmid large-scale extraction kit.
[0215] The amino acid sequence of the chimeric antigen receptor (CAR) is shown in SEQ ID No:21.
[0216]
[0217] in,
[0218] The CD8 leader peptide is the 1st-21st po...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com