Cannabidiol nasal preparation for treating post-traumatic stress disorder
A post-traumatic stress and cannabidiol technology, applied in the field of cannabidiol preparations for the treatment of post-traumatic stress disorder, can solve problems such as blood and lymphatic system discomfort, side effects that cannot be ignored, and immune disorders
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Cannabidiol nasal thermosensitive gel
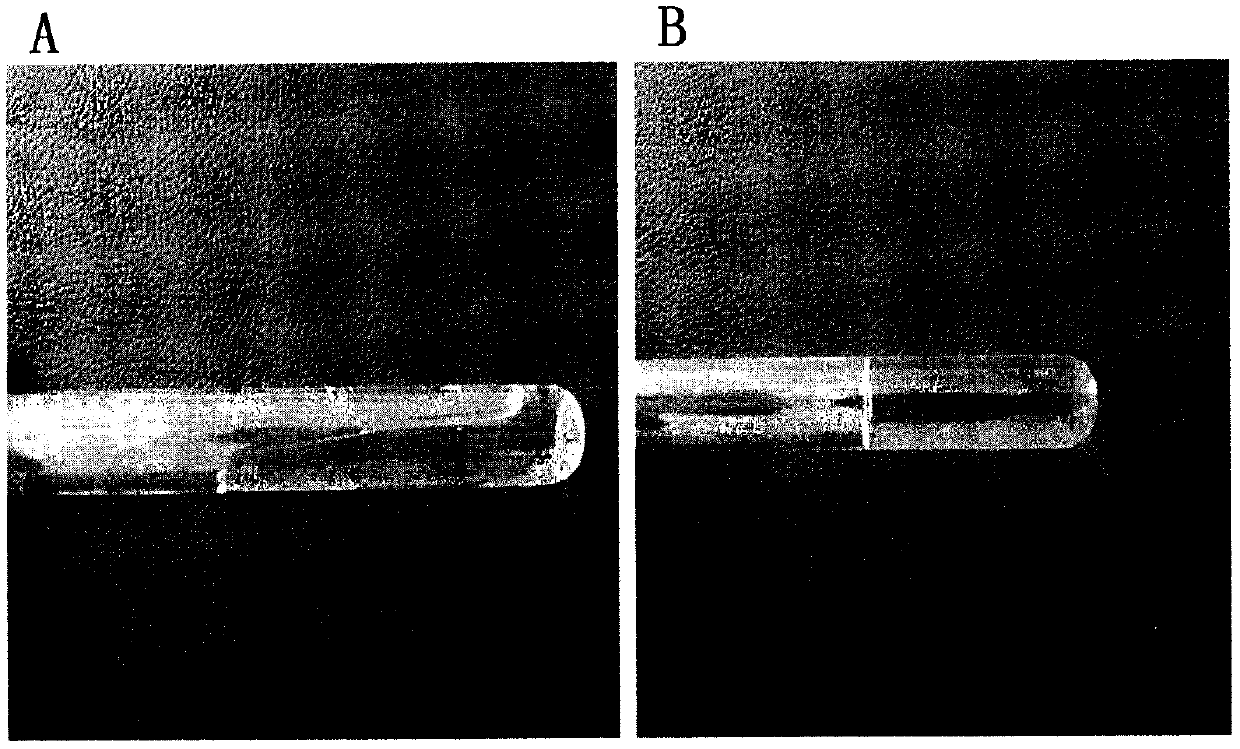
[0032] Take 2g of poloxamer 188 and 12g of poloxamer 407, add appropriate amount of water to fully swell at 4°C, continue to add water to 50ml, and mix well to obtain a blank thermosensitive gel; take 1g of cannabidiol, add 6mL of 1,2-propanediol and 8mL ethanol to make it fully dissolved; add the cannabidiol solution to 36mL of the above-mentioned blank temperature-sensitive gel, and mix well to obtain a cannabidiol nasal preparation. At 4°C, the appearance of the nasal temperature-sensitive gel composition is colorless, transparent and viscous solution, which turns into a gel state at 35°C ( figure 1 ).
experiment example 1
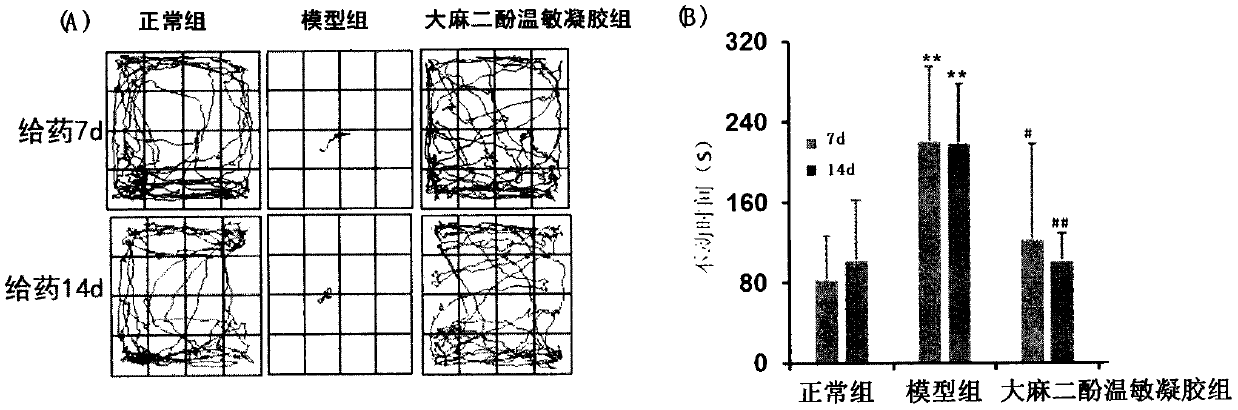
[0033] Experimental Example 1: Pharmacodynamic evaluation of cannabidiol nasal thermosensitive gel in the treatment of post-traumatic stress disorder
[0034] 1 Materials and methods:
[0035] 1.1 Experimental animals and grouping: Male C57 mice, SPF grade, 20±1g, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., were randomly divided into normal group, model group, and cannabidiol thermosensitive gel group, each group 3 Only.
[0036] 1.2 Construction of animal model: the mice in the normal group were placed in the conditioned fear box to move freely for 600s without any stimulation, and the mice in the model group and the administration group were stimulated as follows: set the current to 0.8mA, put the mice in the fear box Within 300s of free movement, the electric stimulation stage was entered, which lasted for 10s, with an interval of 10s, and 15 electric shocks. After each animal experiment, spray alcohol on a paper towel and wipe it clean t...
Embodiment 2
[0047] Example 2 Cannabidiol, hydroxypropyl-β-cyclodextrin inclusion compound composition
[0048] Accurately weigh 0.5g of hydroxypropyl-β-cyclodextrin in a 50mL beaker, add 10mL of ultrapure water and stir to dissolve to obtain 50mg·mL -1 Hydroxypropyl-β-cyclodextrin solution; accurately weigh 0.1g of cannabidiol in a centrifuge tube, add 10mL of tert-butanol solution and ultrasonically dissolve to obtain 10mg·mL -1 Cannabidiol tert-butanol solution; slowly inject the cannabidiol tert-butanol solution into the hydroxypropyl-β-cyclodextrin solution under magnetic stirring conditions, mix well, and freeze-dry in a freeze dryer for 42 hours. Obtain cannabidiol hydroxypropyl-β-cyclodextrin inclusion compound. Infrared results proved that after cannabidiol formed an inclusion compound, the characteristic absorption peak disappeared ( Figure 5 ), proving that cannabidiol was successfully encapsulated into the cavity of hydroxypropyl-β-cyclodextrin. And as the concentration of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com