A method for efficiently treating cr-containing 6+ (b,o) co-doping g-c of wastewater 3 no 4 Preparation method of photocatalyst
A photocatalyst, g-c3n4 technology, used in physical/chemical process catalysts, chemical instruments and methods, special compound water treatment, etc. The effect of simple process and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] First, weigh 10g of urea with an electronic analytical balance, and then weigh 0.1g of NaBH 4 (i.e. urea and NaBH 4 The mass ratio is 100:1), put it into a mortar, grind for 20 minutes to mix all components well, then put it into a 100ml crucible, cover it and heat it in a muffle furnace at a rate of 5°C / min rate, calcined at a high temperature of 550°C for 2h, and took out the sample after naturally cooling to room temperature with the furnace. The samples were centrifuged and washed three times with deionized water, put into a drying oven at 60°C for 24 hours, and finally the catalyst was collected.
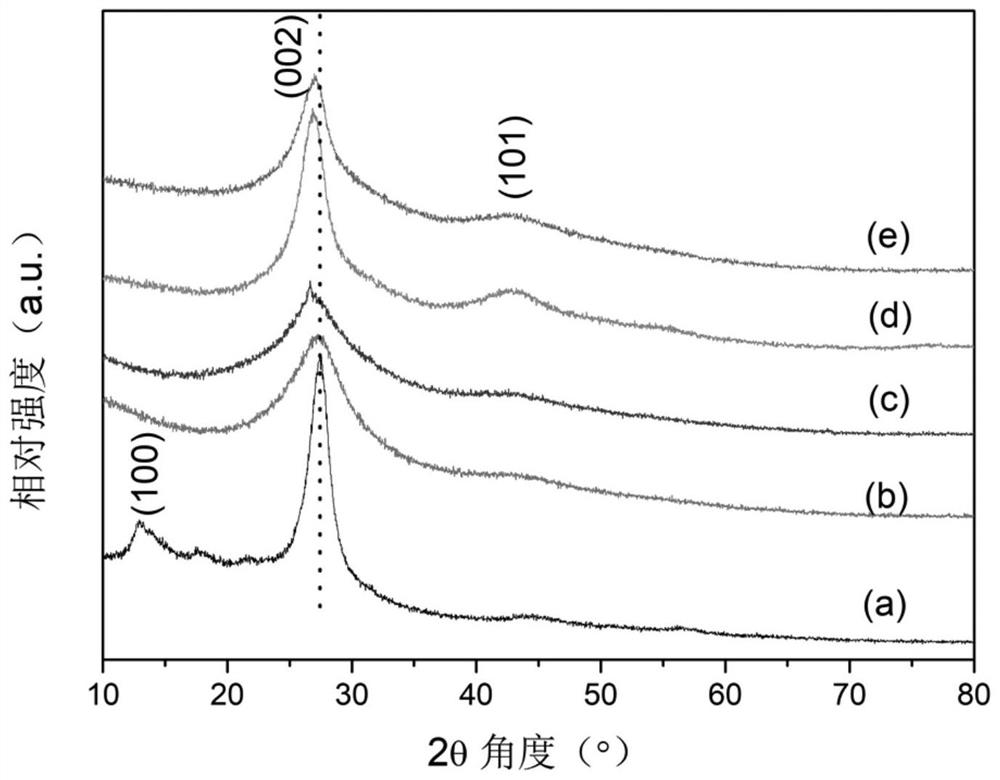
[0044] XRD spectrum ( figure 1 (b)) shows that for the addition of 0.1g of NaBH 4 Modified g-C 3 N 4 , with g-C before modification 3 N 4 In contrast, the diffraction peak corresponding to the (100) crystal plane is missing in the XRD spectrum, which is caused by the preferred orientation of the crystal plane; and the position of the diffraction peak corresponding...
Embodiment 2
[0046] First, weigh 10g of urea with an electronic analytical balance, and then weigh 0.2g of NaBH 4 (i.e. urea and NaBH 4 The mass ratio is 50:1), put it into a mortar, grind for 20 minutes to mix all components well, then put it into a 100ml crucible, cover it and heat it in a muffle furnace at a rate of 5°C / min rate, calcined at a high temperature of 550°C for 2h, and took out the sample after naturally cooling to room temperature with the furnace. The samples were centrifuged and washed three times with deionized water, dried in a drying oven at 60°C for 24 hours, and finally collected.
[0047] XRD spectrum ( figure 1 (c)) shows that for the addition of 0.2g of NaBH 4 Modified g-C 3 N 4 , with g-C before modification 3 N 4 In contrast, the diffraction peak corresponding to the (100) crystal plane is missing in the XRD spectrum, which is caused by the preferred orientation of the crystal plane; and the position of the diffraction peak corresponding to the (002) crys...
Embodiment 3
[0049] First, weigh 10g of urea with an electronic analytical balance, and then weigh 0.4g of NaBH 4 (i.e. urea and NaBH 4 The mass ratio is 25:1), put it into a mortar, grind for 20 minutes to mix all components well, then put it into a 100ml crucible, cover it and heat it in a muffle furnace at a rate of 5°C / min rate, calcined at a high temperature of 550°C for 2h, and took out the sample after naturally cooling to room temperature with the furnace. The samples were centrifuged and washed three times with deionized water, dried in a drying oven at 60°C for 24 hours, and finally collected.
[0050] XRD spectrum ( figure 1 (d)) shows that for the addition of 0.4g of NaBH 4 Modified g-C 3 N 4 , with g-C before modification 3 N 4 In contrast, the diffraction peak corresponding to the (100) crystal plane is missing in the XRD spectrum, and the diffraction peak corresponding to the (101) crystal plane appears near 43°, which is caused by the preferred orientation of the cry...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com