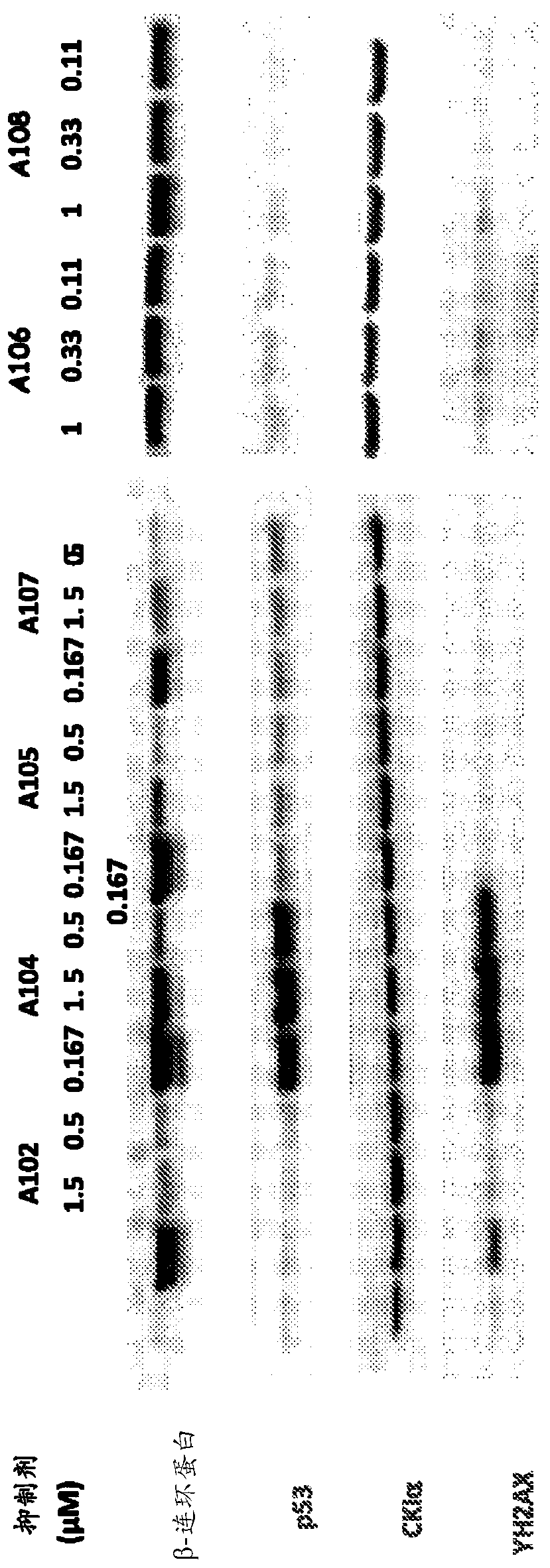
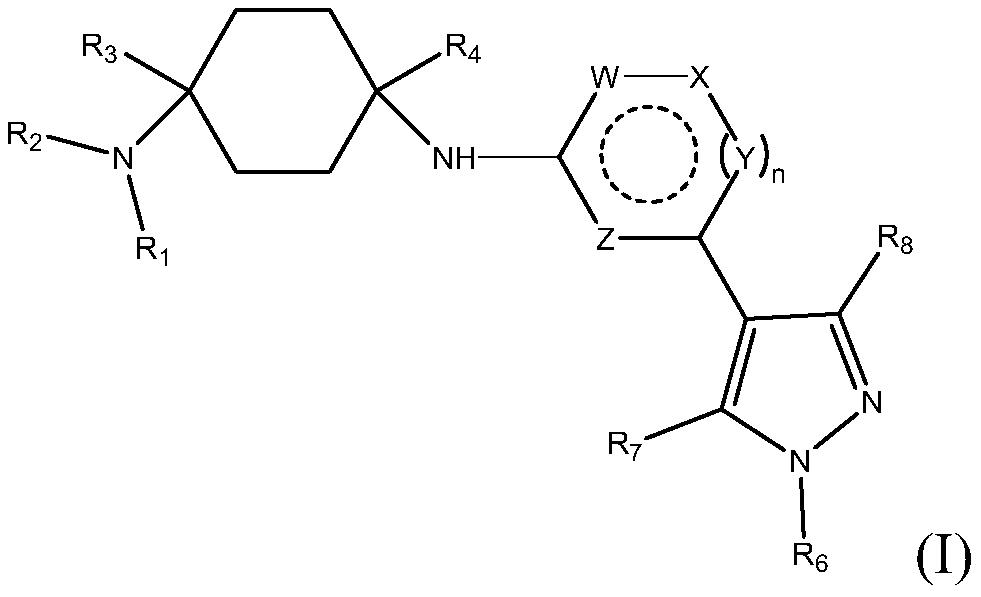
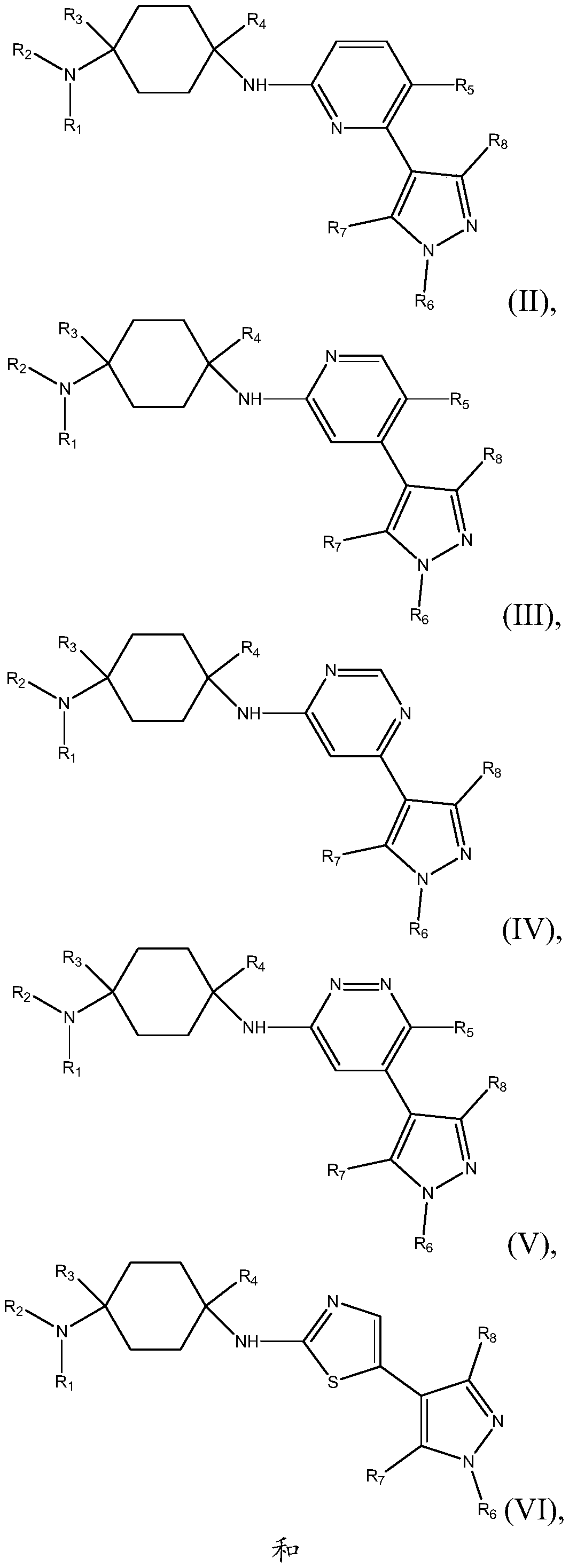
N1-(4-(5-(cyclopropylmethyl)-1 -methyl-1 h-pyrazol-4-yl)pyridin-2-yl)cyclohexane-1,4-diamine derivatives and related compounds as ck1 and/or iraki inhibitors for treating cancer
A technology of C1-C5, C5-C15, applied in a field of use in a method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0197] Example 1: 5-(Cyclopropylmethyl)-1-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxolane-2- (Base)-1H-pyrazole (1) synthesis
[0198]
[0199] Step 1: Cyclopropyl(1-methyl-1H-pyrazol-5-yl)methanol (1-2): Add compound N-methylpyrazole (1-1,8.00g, 97.44mmol, 1.00eq) in THF (160mL) was added dropwise n-BuLi (2.5M, 46.77mL, 1.20eq). After 1 hour at -78°C, a solution of cyclopropanecarboxaldehyde (8.20 g, 116.93 mmol, 1.20 eq) in THF (80 mL) was added dropwise. Stir the resulting mixture at 20°C for 16 hours and pour into NH 4 Cl aqueous solution (300 mL), and stir for 10 minutes. The aqueous phase was extracted with ethyl acetate (100 mL×2). The combined organic phase was washed with brine (100 mL), and subjected to anhydrous Na 2 SO 4 Dry, filter and concentrate in vacuo. Column chromatography (SiO 2 ) The residue was purified to obtain compound 1-2 (12.00 g, 78.85 mmol, yield 80.9%, purity 100%) as a colorless oil. LCMS: RT=0.118min, m / z 153.1[M+H] + .
[0200] Step 2: 5-(Cyclop...
Embodiment 2
[0203] Example 2: (1r, 4r)-N1-(5-chloro-4-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridin-2-yl) Synthesis of cyclohexane-1,4-diamine (A104)
[0204]
[0205] Step 1: 2,5-Dichloro-4-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridine (104-2): to 2,5-di Chloro-4-iodopyridine (104-1, 523.13mg, 1.91mmol, 1.00eq) and compound 1 (500.8mg, 1.91mmol, 1.0eq) are added to the mixture in DME (10mL) 2 CO 3 (2M, 2.87mL, 3.00eq) and bis(di-tert-butyll(4-dimethylaminophenyl)phosphine)dichloropalladium(II) (67.62mg, 95.50μmol, 67.62μL, 0.05eq). The resulting mixture was stirred at 80°C under nitrogen for 2 hours, cooled to room temperature, concentrated, and purified by column chromatography to obtain 104-2 (200 mg, 659.3 μmol, yield 34.5%, purity 93.0) as a yellow oil %). LCMS: RT=0.825min, m / z282.0[M+H] + .
[0206] Step 2: (1r, 4r)-N1-(5-chloro-4-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridin-2-yl) ring Hexane-1,4-diamine (A104): To 104-2 (180.00mg, 637.91μmol, 1.00eq)...
Embodiment 3
[0207] Example 3: (1r,4r)-N1-(6-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridin-2-yl)cyclohexane- Synthesis of 1,4-diamine (A105)
[0208]
[0209] Step 1: 2-Chloro-6-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridine (105-2): To 2,6-dichloropyridine ( 562mg, 3.81mmol, 1.00eq) and compound 1 (1.00g, 3.81mmol, 1.0eq) in DME (20mL) was added Na 2 CO 3 (2M, 5.72mL, 3.00eq) and bis(di-tert-butyl(4-dimethylaminophenyl)phosphine)dichloropalladium(II) (134.89mg, 190.5umol, 134.89uL, 0.05eq). The mixture was stirred at 80°C for 2 hours, cooled to room temperature, concentrated, and purified by column chromatography to obtain 105-2 (500 mg, 1.32 mmol, yield 34.6%, purity 74.5%) as a yellow oil. LCMS: RT=0.835min, m / z 248.1[M+H] + .
[0210] Step 2: (1r,4r)-N1-(6-(5-(cyclopropylmethyl)-1-methyl-1H-pyrazol-4-yl)pyridin-2-yl)cyclohexane-1 ,4-Diamine (A105): To 105-2 (400.00mg, 1.61mmol, 1.00eq) and (1r,4r)-cyclohexane-1,4-diamine (275.77mg, 2.42mmol, 1.50eq) Add tertiary BuON...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com