Chimeric antigen receptors targeting tim-1
A chimeric antigen receptor, TIM-1 technology, applied in the field of preparation and use of such compositions, can solve problems such as not yet achieved success
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0289] In some embodiments, the method of preparation includes the step of freezing (eg, cryopreserving) the cells before or after isolation, incubation, and / or engineering. In some embodiments, the step of freezing followed by thawing removes granulocytes, and to some extent monocytes, from the cell population. In some embodiments, cells are suspended in a freezing solution to remove plasma and platelets, eg, after a washing step. In some aspects, any of a variety of known freezing solutions and parameters can be used. One example involves the use of PBS containing 20% DMSO and 8% human serum albumin (HSA), or other suitable cell freezing medium. Then it was diluted 1:1 with culture medium so that the final concentrations of DMSO and HSA were 10% and 4%, respectively. Cells were then frozen to -80°C at a rate of 1°C per minute and stored in the gas phase of liquid nitrogen tanks.
[0290] therapeutic application
[0291] Isolated cells obtained by the methods describe...
Embodiment 1
[0409] Example 1: Design and synthesis of anti-TIM-1 CAR variants
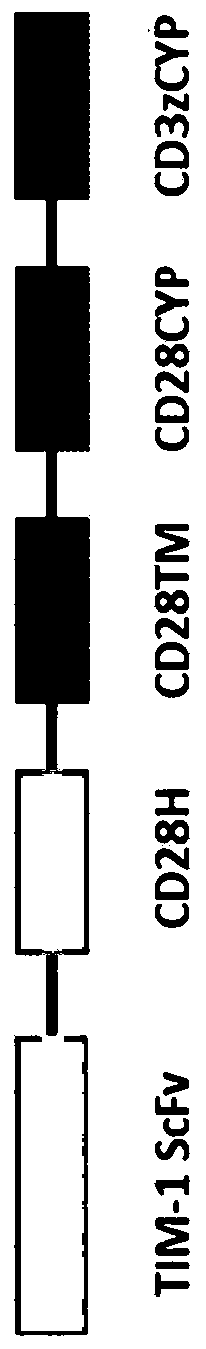
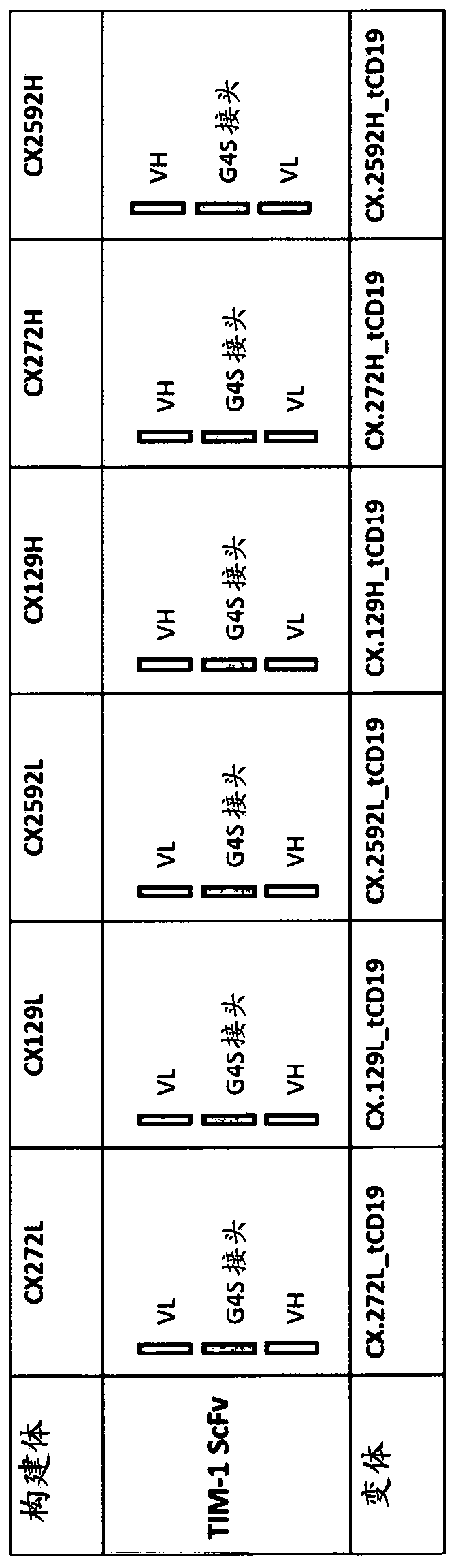
[0410] according to figure 1 General schematic for generation of anti-TIM-1 CAR. For the six variants, Figure 2A A more detailed schematic of the CAR construct is provided. The CAR structure is based on a second generation CAR format (Gacerez et al., J Cell Physiol, 2016 Dec;231(12):2590-8). There are six different TIM-1 -responsive single change variable fragments (scFv) in Hv-linker-Lv or Lv-linker-Hv orientation from anti-TIM1 hybridoma clones 1.29, 2.70.2 or 2.59.2 ( Figure 2B ). These anti-TIM-1 scFvs were formed by fusing the variable domains of the heavy chain (VH) and light chain (VL) domains to the following 15 amino acid glycine (G)-serine (S) linker: (G4S)3 Linker (SEQ ID NO:201), 3 repeats of GGGGS (SEQ ID NO:200). These were cloned individually in frame into the hinge domain containing CD28 (residues 135-152 of CD28 or SEQ ID NO:214), the transmembrane domain of CD28 (residues 153-179 of ...
Embodiment 2
[0417] Example 2: Generation of anti-TIM-1 CAR T cells
[0418] Cell culture and retroviral transduction: The retroviral stocks described above were used using optimized methods (Cubillos-Ruiz et al., Oncotarget 2010; 1:329-33; Huarte et al., Blood 2008; 112:1259-1268 Stephen et al., Immunity 2014;41:427-439), protocols and resources previously developed at Celdara Medical to transduce human T cells from healthy donors.
[0419] For some variants, cell culture, retroviral transduction and purification protocols are summarized in image 3 middle. The resulting transduced cells were analyzed by flow cytometry ( Figure 4 ). For the purification of some variants, the protocol is as follows. Human PBMCs from healthy donors (HemaCare) were the source of T cells for CAR transduction. Donor PBMCs were thawed, reconstituted in complete medium, and pelleted by centrifugation. Cells were then resuspended in complete medium and then activated by incubation with IL-2 and anti-CD3 fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com