Zinc borate, flame-retardant composite material and preparation mode of zinc borate
A technology of composite materials and zinc borate, which is applied in the field of inorganic functional materials science, can solve the problems of deterioration of mechanical properties of polymer composite materials, poor compatibility of substrates, difficulty in uniform dispersion, and large size of zinc borate products, so as to improve mechanical properties and Flame retardant performance, high product quality, and the effect of increasing the contact area of the two phases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 3ZnOB 6 o 12 3.5H 2 O preparation
[0024] Add 40mL of solvent water into the reactor at normal pressure, heat and stir in an oil bath at 100°C, add 5.26g of zinc oxide under the state of boiling and refluxing the solvent water to form a heterogeneous solution system, stir at 200r / min for 5min, and then add 20.0g boric acid. The ratio of boric acid to zinc oxide is 5:1, and the ratio of boric acid to water is 2:1. After continuing the reaction for 3 hours, filter the obtained product and wash it with hot water above 90°C to obtain zinc borate 3ZnOB 6 o 12 3.5H 2 O, the sample was air-dried at 80°C to obtain 3ZnOB 6 o 12 3.5H 2 O product 15.5g.
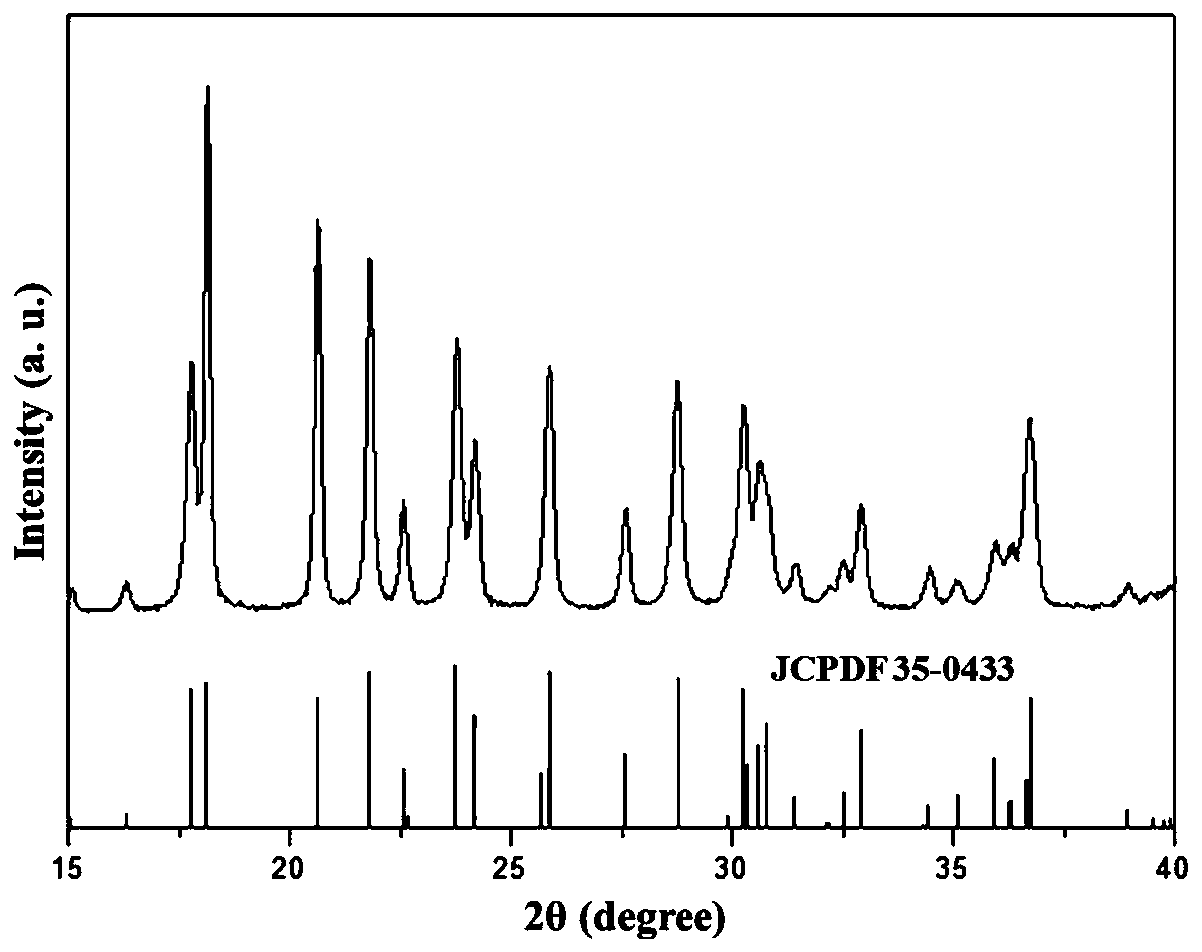
[0025] figure 1 For the XRD spectrum of the product prepared in Example 1, it can be seen from the figure that all diffraction peaks belong to the JCPDS 35-0433 standard 3ZnOB 6 o 12 3.5H 2 O diffraction peak, and no other impurity peaks appear.
Embodiment 2
[0026] Example 2 3ZnOB 6 o 12 3.5H 2 O preparation
[0027] Add 50mL of solvent water into the reactor at normal pressure, heat and stir in an oil bath at 150°C, add 3.75g of zinc oxide into the solvent water under vigorous boiling and reflux to form a heterogeneous solution system, stir at 400r / min for 5min, and then add 20.0 g boric acid. The ratio of boric acid to zinc oxide is 7:1, and the ratio of boric acid to water is 2.5:1. After continuing the reaction for 1 hour, filter the obtained product and wash it with hot water above 90°C to obtain zinc borate 3ZnOB 6 o 12 3.5H 2 O, the sample was air-dried at 80°C to obtain 3ZnOB 6 o 12 3.5H 2 O product 12.9 g.
Embodiment 3
[0028] Example 3 3ZnOB 6 o 12 3.5H 2 O preparation
[0029] Add 40mL of solvent water into the reactor at normal pressure, heat and stir in an oil bath at 100°C, add 4.38g of zinc oxide into the water under the state of vigorous boiling and reflux of the solvent water, stir at 300r / min for 5min, then add 20.0g of boric acid, The ratio of boric acid to zinc oxide is 6:1, and the ratio of boric acid to water is 2.25:1. After continuing the reaction for 1.5 hours, filter the obtained product and wash it with hot water above 90°C to obtain zinc borate 3ZnOB 6 o 12 3.5H 2 O, the sample was air-dried at 60°C to obtain 3ZnOB 6 o 12 3.5H 2 O product 13.9 g.
[0030] figure 2 3ZnOB prepared for Example 3 6 o 12 3.5H 2 Transmission electron microscope image of O; image 3 It is a scanning electron microscope picture of Example 3, showing that the product size is 50-100nm and the thickness is about 10-20nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com