Method for preparing cubic precipitated calcium carbonate
A technology of precipitated calcium carbonate and cubic shape, applied in the field of calcium carbonate, which can solve problems such as difficult quality control, complicated preparation process, and increased cost, and achieve the effects of good quality control, simple process, and control of production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Add 2500 grams of quicklime to 40 liters of water at 48°C and stir for 30 minutes to make a calcium hydroxide slurry, and then pass it through a 200-mesh sieve for later use; the concentration of the obtained calcium hydroxide slurry is 61.3 g / L;
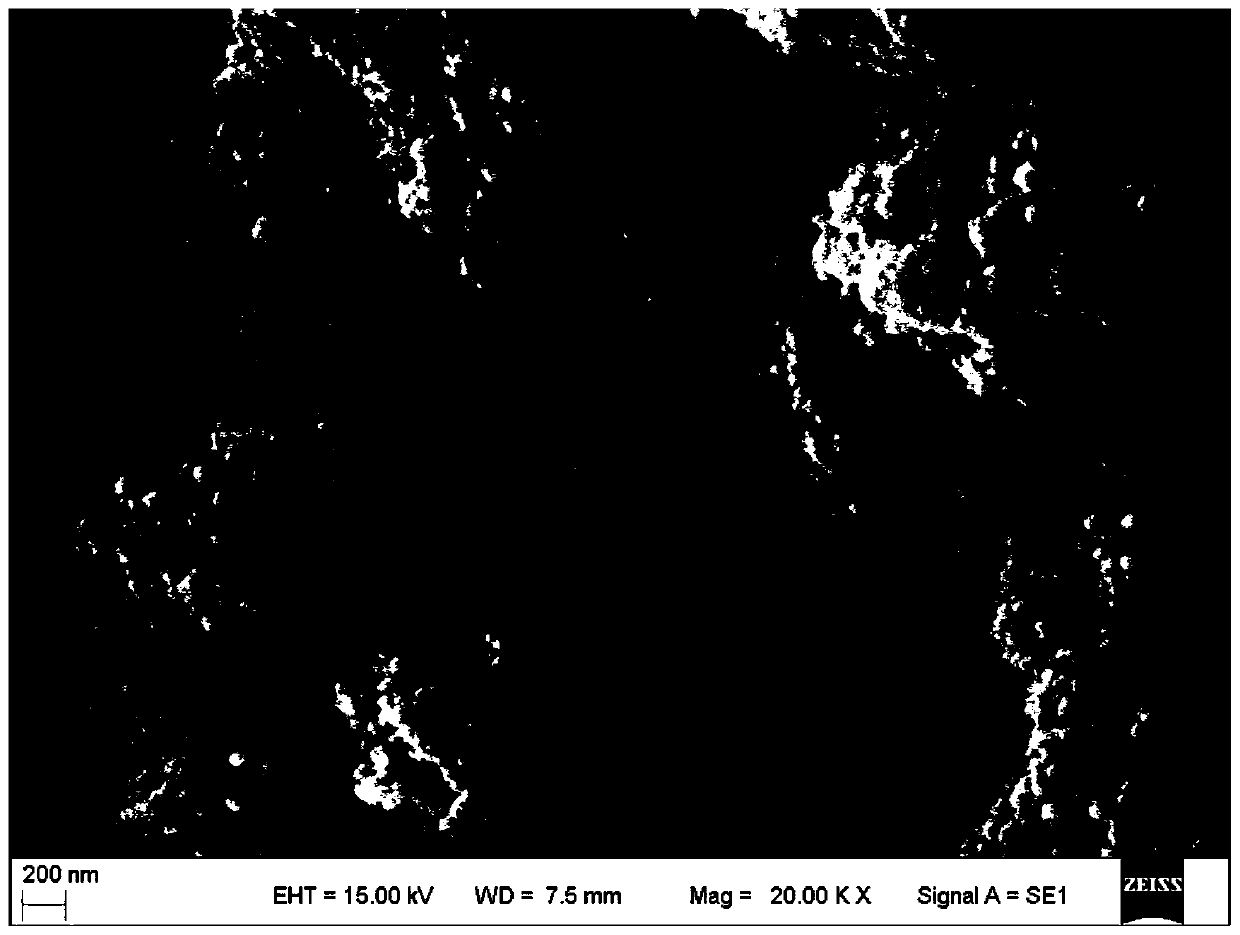
[0024] (2) Add the above-mentioned 30 liters of calcium hydroxide slurry into a jacketed reaction vessel equipped with a dispersing paddle; open the dispersing paddle (linear velocity 5.6 m / s), and fill the jacket with circulating cooling water to make the reaction vessel The calcium hydroxide slurry is cooled to 11.3°C, and then filled with carbon dioxide at a rate of 29.4 liters / minute; simultaneously, the temperature of the slurry in the reaction vessel is controlled at 10-25°C until the pH value of the slurry in the reaction vessel reaches 6.2; the reaction is completed and obtained Aqueous slurry of cubic precipitated calcium carbonate, products such as figure 1 shown.
[0025] Depend on figure 1 It can be seen tha...
Embodiment 2
[0027] (1) Add 1900 grams of quicklime to 30 liters of water at 48°C and stir for 30 minutes to make a calcium hydroxide slurry, and then pass it through a 200-mesh sieve for later use; the concentration of the obtained calcium hydroxide slurry is 52.9 grams per liter;
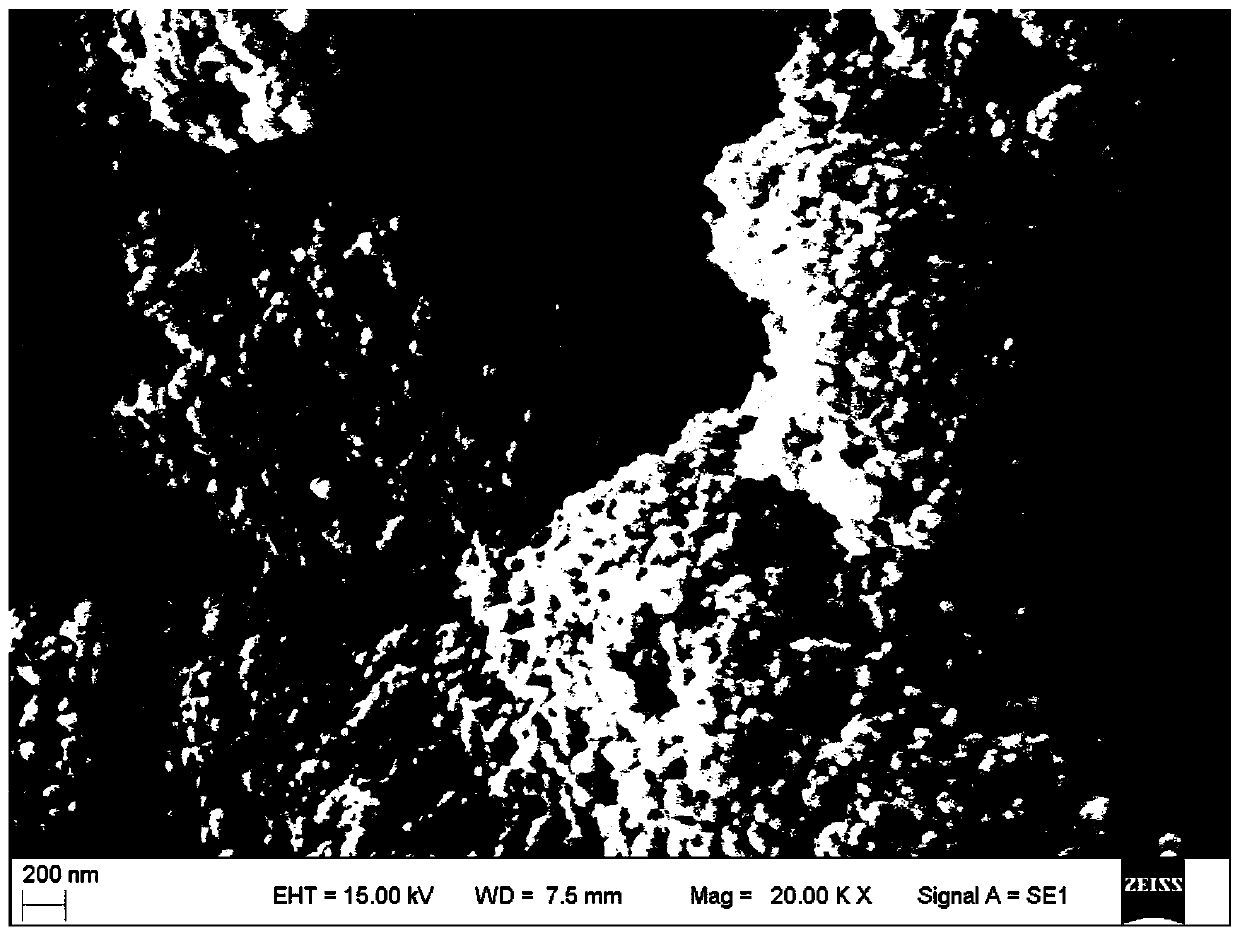
[0028] (2) Add the above-mentioned 28 liters of calcium hydroxide slurry into a jacketed reaction vessel equipped with a dispersing paddle; open the dispersing paddle (linear velocity 5.6 m / s), and fill the jacket with circulating cooling water to make the reaction vessel The calcium hydroxide slurry is cooled to 15.2°C, and then filled with carbon dioxide at a rate of 23.7 liters / minute; simultaneously, the temperature of the slurry in the reaction vessel is controlled at 14-22°C until the pH value of the slurry in the reaction vessel reaches 6.2; the reaction is completed and obtained Aqueous slurry of cubic precipitated calcium carbonate, products such as figure 2 shown.
[0029] Depend on figure 2 It c...
Embodiment 3
[0031] (1) Add 2506 grams of quicklime to 30 liters of water at 43°C and stir for 30 minutes to make a calcium hydroxide slurry, and then pass it through a 200-mesh sieve for later use; the concentration of the obtained calcium hydroxide slurry is 59.2 grams per liter;
[0032] (2) Add the above-mentioned 28 liters of calcium hydroxide slurry into a jacketed reaction vessel equipped with a dispersing paddle; open the dispersing paddle (linear velocity 5.6 m / s), and fill the jacket with circulating cooling water to make the reaction vessel The calcium hydroxide slurry is cooled to 16.8°C, and then filled with carbon dioxide at a rate of 26.5 liters / minute; simultaneously, the temperature of the slurry in the reaction vessel is controlled at 16-25°C until the pH value of the slurry in the reaction vessel reaches 6.0; the reaction is completed, and the obtained Aqueous slurry of cubic precipitated calcium carbonate, products such as image 3 shown.
[0033] Depend on image 3 I...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com