Mutant of human papilloma virus 39 type L1 protein
A protein and variant technology, applied in the fields of molecular virology and immunology, can solve problems such as safety issues and increased production costs of HPV vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
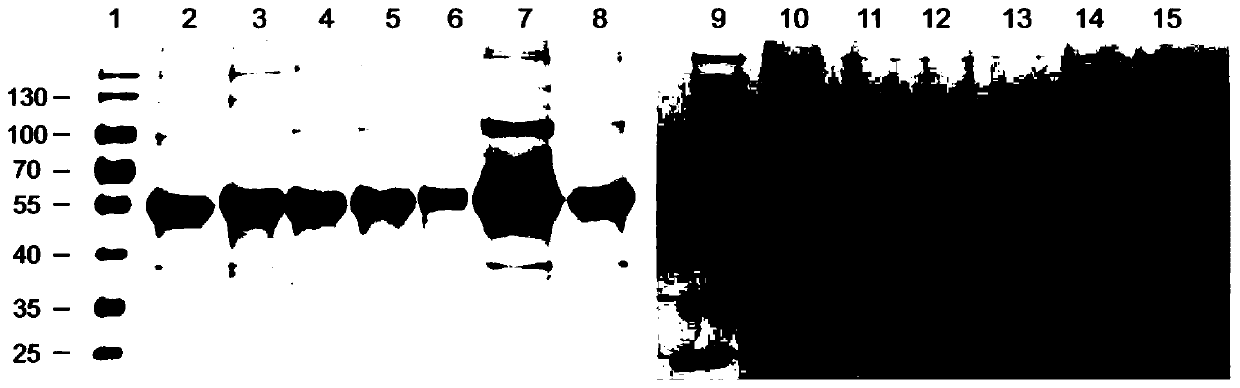
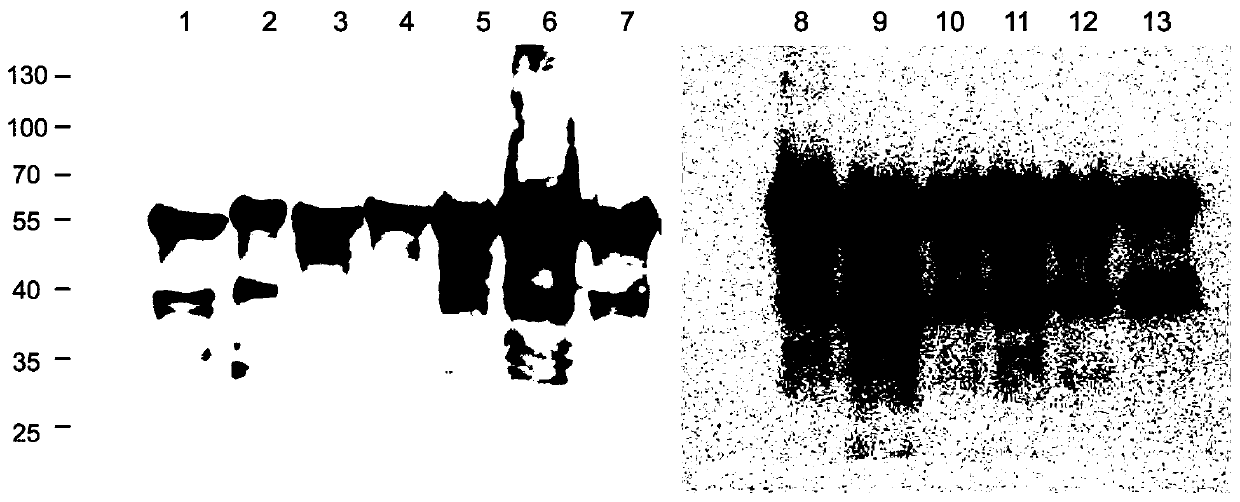
[0165] Example 1. Expression and purification of mutant HPV39 L1 protein
[0166] Construction of expression vector
[0167] A multi-point mutation PCR reaction was used to construct an expression vector encoding a mutant HPV39L1 protein containing a segment derived from the HPV68L1 protein. The initial template used was the pTO-T7-HPV39N15C plasmid (which encodes 15 truncated N-terminals). HPV39 L1 protein of amino acids; abbreviated as 39 L1N15 in Table 2). The templates and primers used in each PCR reaction are shown in Table 2, and the amplification conditions of the PCR reaction are set to: denaturation at 94°C for 10 minutes; 25 cycles (denaturation at 94°C for 50 seconds, annealing at specified temperature for a certain time, extension at 72°C 7 minutes 30 seconds); the final extension at 72°C for 10 minutes. The annealing temperature and time are listed in Table 2. The specific sequences of the PCR primers used are listed in Table 3.
[0168] Add 2 μL of DpnI restriction...
Embodiment 2
[0195] Example 2: Assembly of HPV virus-like particles and particle morphology detection
[0196] Assembly of HPV virus-like particles
[0197] Take a certain volume (about 2ml) of protein H39N15-68T1, H39N15-68T2, H39N15-68T3, H39N15-68T4, H39N15-68T5, H39N15-68T4-70S1, H39N15-68T4-70S2, H39N15-68T4-70S3 and H39N15-68T4-70S68 -70S5, respectively dialyzed to (1) 2L storage buffer (20mM sodium phosphate buffer pH 6.5, 0.5M NaCl); (2) 2L refolding buffer (50mM sodium phosphate buffer pH 6.0, 2mM CaCl) 2 , 2mM MgCl 2 , 0.5M NaCl); and (3) 20mM sodium phosphate buffer pH 7.0, 0.5M NaCl. Dialysis was performed in each of the three buffers for 12 h.
[0198] In a similar way, HPV39N15, HPV68N0 and HPV70N10 proteins were assembled into HPV39N15VLP, HPV68N0 VLP and HPV70N10 VLP, respectively.
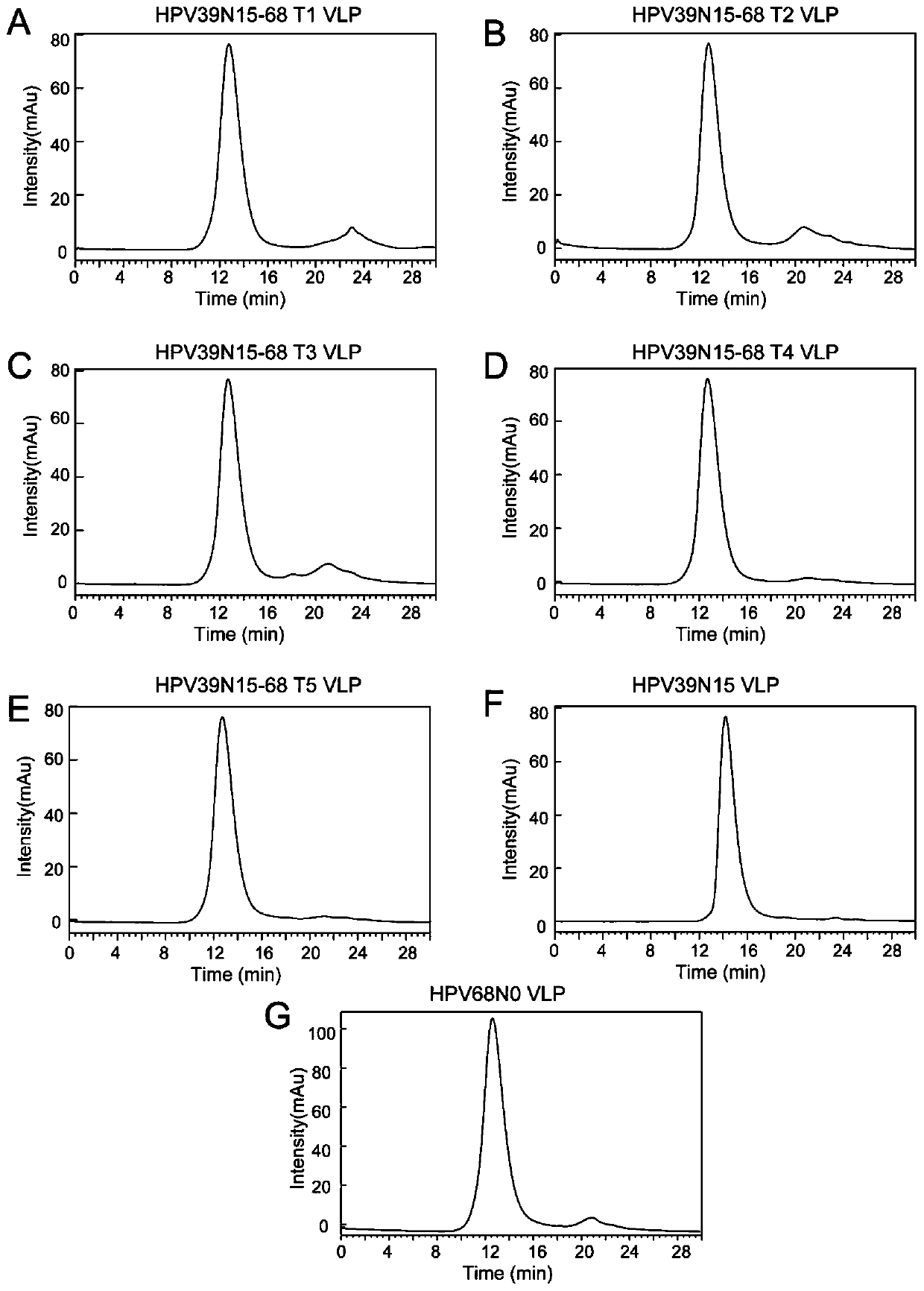
[0199] Molecular Sieve Chromatography Analysis
[0200] Molecular sieve chromatography was performed on the dialyzed samples with the 1120Compact LC high performance liquid chromatography system of A...
Embodiment 3
[0205] Example 3: Evaluation of neutralizing antibody titer in mouse serum after immunization with virus-like particles 1
[0206] In this experiment, the virus-like particles used are: H39N15-68T1 VLP, H39N15-68T2 VLP, H39N15-68T3VLP, H39N15-68T4 VLP and H39N15-68T5 VLP.
[0207] In this experiment, the immunization scheme is shown in Table 4. Divide all mice (6-week-old BalB / c female mice) into 3 groups: aluminum adjuvant group 1 (immunization dose is 5μg, using aluminum adjuvant), aluminum adjuvant group 2 (immunization dose is 1μg, using aluminum adjuvant) Aluminum adjuvant), and aluminum adjuvant group 3 (immunization dose is 0.2 μg, using aluminum adjuvant). Each group was subdivided into 8 subgroups. Control subgroups 1 and 2 were immunized with HPV39N15 VLP alone and HPV68N0 VLP alone, and control subgroup 3 was immunized with mixed HPV39 / HPV68VLP (ie, HPV39N15 VLP and HPV68N0 VLP). A mixture in which each VLP is administered at a specified immunizing dose) for immunizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| radius | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com