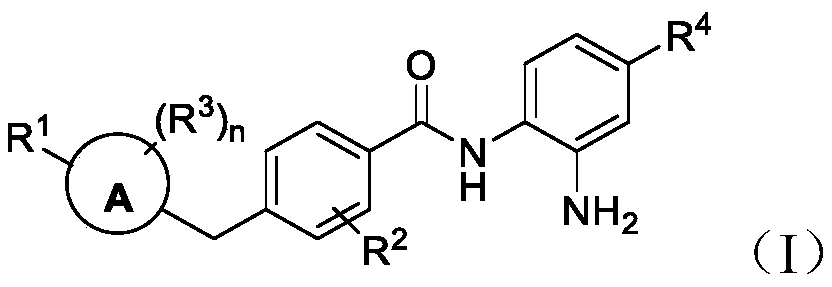
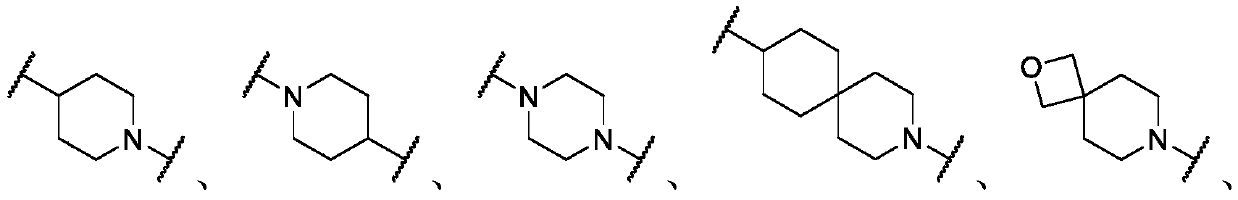
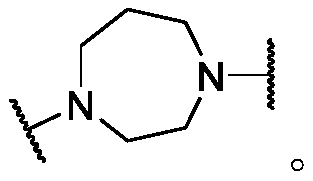
Histone deacetylase inhibitors
An alkyl and alkenyl technology, applied in the field of compounds that inhibit histone deacetylase enzymes, can solve the problem of less specific isomer effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0111] The synthesis of specific compounds was carried out as follows.
[0112] abbreviation
[0113]
[0114]
[0115] Synthetic scheme of compound 485 and compound 486:
[0116]
[0117] Step 1: Synthesis of tert-butyl 4-(4-(methoxycarbonyl)benzyl)-1,4-diazepane-1-carboxylate (3): To compound 1 (1.92g, 1.1eq .) and a stirred solution of compound 2 (2 g, 1 eq.) in ACN (20 mL) was added potassium carbonate (1.8 g, 1.5 eq.). The reaction mixture was stirred at room temperature for 16 h. The reaction was monitored for completion by TLC. The reaction mixture was diluted with water and extracted with ethyl acetate. The combined organic extracts were washed with water, brine, and washed with anhydrous Na2 SO 4 Drying, filtration and concentration under reduced pressure afforded the title compound 3 which was used in the next step without further purification.
[0118] Step 2: Synthesis of 4-((1,4-diazepan-1-yl)methyl)benzoic acid methyl ester hydrochloride (4): at 0°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com