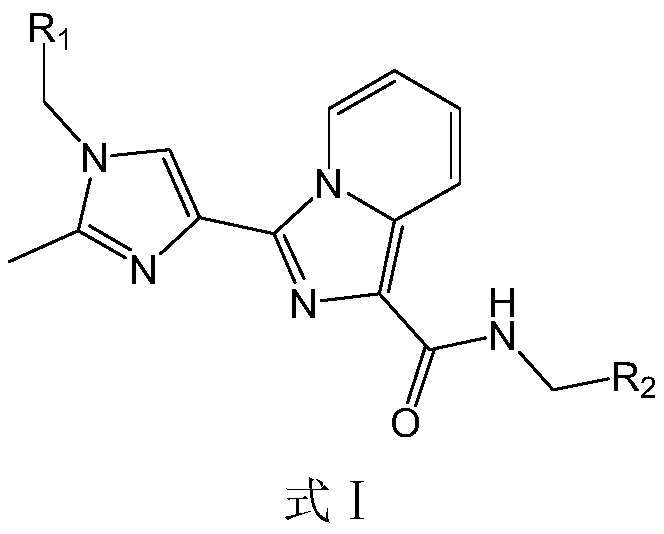
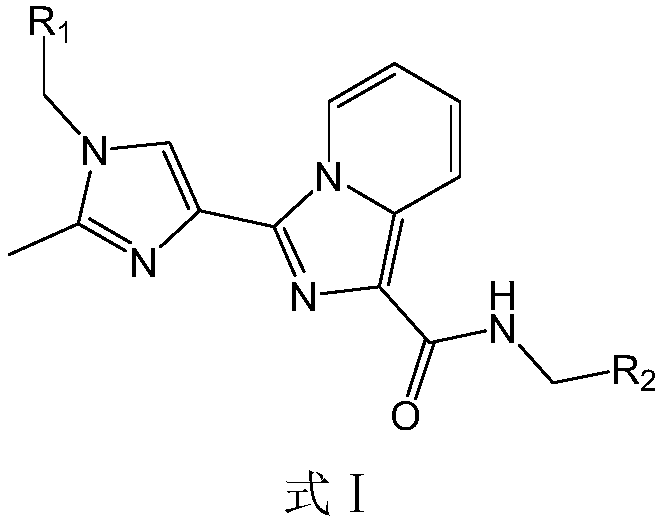
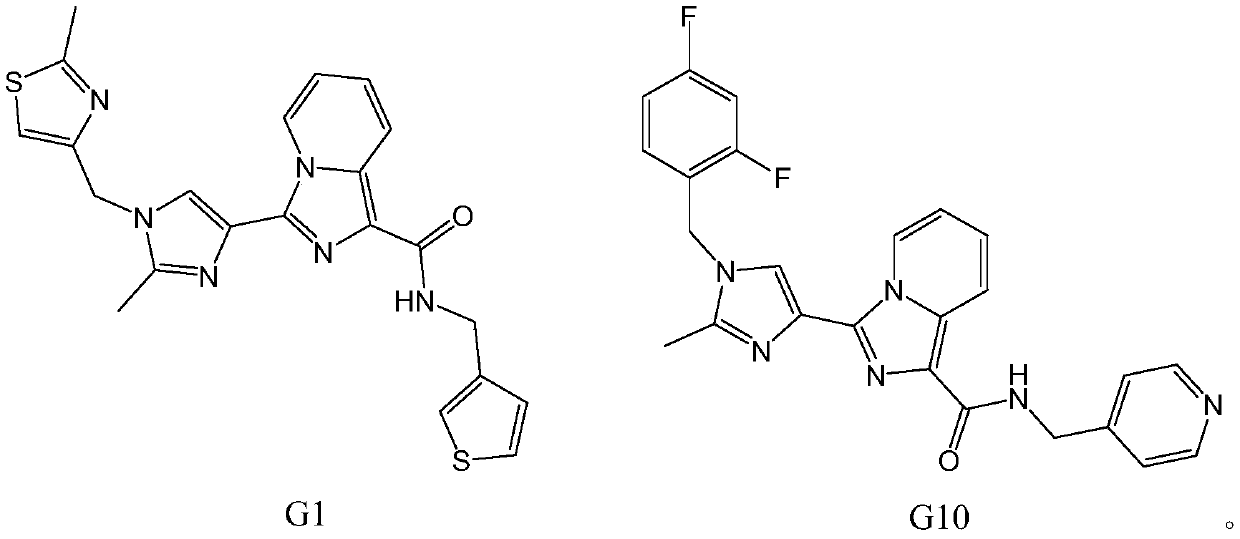
HDAC6 selective inhibitor
A selective and inhibitory technology, applied in the field of anti-tumor drugs, can solve problems such as strong toxic and side effects, and achieve the effect of high inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 HDACs inhibitory activity
[0054] Inhibitory activity of G1 and G10 compounds on HDAC6: Compound G1 or G10 at a concentration of 20 μM was incubated with recombinant HDAC6 (BPS Biosciences, US) for 15 minutes at room temperature, and then the substrate Ac-peptide-AMC (7-amino-4- Coumarin-labeled acetylated peptide, Jill Biochemical) started the reaction. The reaction was carried out at room temperature for 60 minutes, then a trypsin solution was added to stop the reaction, and the reaction solution was stored at 37° C. for another 90 minutes. The fluorophore AMC released from the substrate was measured with Synergy2 (Biotek, US, excitation=355nm, emission=460nm). ,
[0055] Use Synergy to select the linear response segment to obtain the slope (slope). Then calculate the percentage inhibition rate, the calculation formula is as follows:
[0056]
[0057] Among them: Inhibition is the inhibition rate; Sample Signal is the slope of the compound hole; Mean...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com