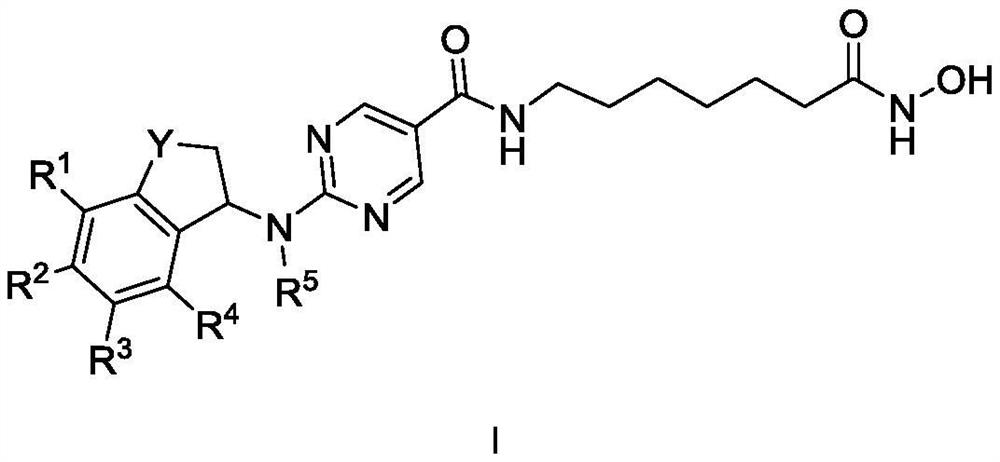
Indane amine derivative, and preparation method and application thereof
A technology of indane amine and compound, which is applied in the field of indane amine derivatives, can solve the problems of inconvenience in clinical use, poor cell metabolism and the like, and achieves the effects of good inhibitory activity, good selectivity and good metabolic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Example 1: N-(7-(hydroxylamino)-7-oxoheptyl)-2-((1,2,3,4-tetrahydronaphthalen-1-yl)amino)pyrimidine-5-carboxamide
[0175]
[0176] Step 1: Synthesis of ethyl 2-((1,2,3,4-tetrahydronaphthalen-1-yl)amino)pyrimidine-5-carboxylate
[0177] Dissolve ethyl 2-chloropyrimidine-5-carboxylate (300mg, 1.61mmol) in N,N-dimethylformamide (5ml), add 1,2,3,4-tetrahydronaphthalene-1-amine (237mg, 1.61mmol) and potassium carbonate (666mg, 4.83mmol). The reaction mixture was stirred at 80°C for 3 hours. The reaction was cooled to room temperature, the mixture was poured into water (50ml), the precipitated solid was filtered, the filter cake was washed with water (20ml), and the filter cake was dried under reduced pressure to obtain the target compound (490mg, crude product, yellow solid). LC-MS(ESI)m / z[M+H] + 298.2.
[0178] Step 2: Synthesis of 2-((1,2,3,4-tetrahydronaphthalen-1-yl)amino)pyrimidine-5-carboxylic acid
[0179] Dissolve ethyl 2-((1,2,3,4-tetrahydronaphthalen-1-yl)...
Embodiment 2
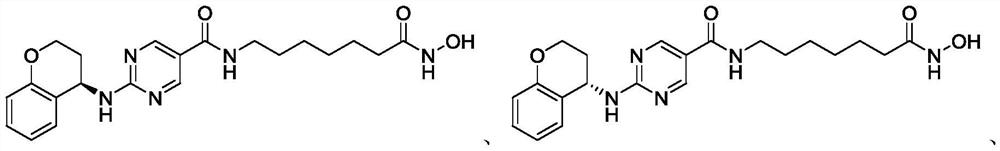
[0184] Example 2: N-(7-hydroxylamino-7-oxoheptyl)-2-(chroman-4-ylamino)pyrimidine-5-carboxamide
[0185]
[0186] Step 1: Synthesis of ethyl 2-(chroman-4-yl-amino)pyrimidine-5-carboxylate
[0187] Ethyl 2-chloropyrimidine-5-carboxylate (200mg, 1.08mmol) and chroman-4-ylamine (240mg, 1.61mmol) were dissolved in 1,4-dioxane (5ml) , triethylamine (326 mg, 3.23 mmol) was added dropwise to the reaction mixture. After the dropwise addition, the reaction mixture was stirred at room temperature for 2 hours. The organic solvent was removed by concentration under reduced pressure, and methanol (2 ml) was added. After filtration, the filter cake was dried under reduced pressure to obtain the target compound (265 mg, yield 82.1%, white solid). LC-MS(ESI)m / z[M+H] + 300.1.
[0188] Step 2: Synthesis of 2-(chroman-4-ylamino)pyrimidine-5-carboxylic acid
[0189] Dissolve ethyl 2-(chroman-4-ylamino)pyrimidine-5-carboxylate (150mg, 0.501mmol) in a mixed solvent of tetrahydrofuran (3ml)...
Embodiment 3
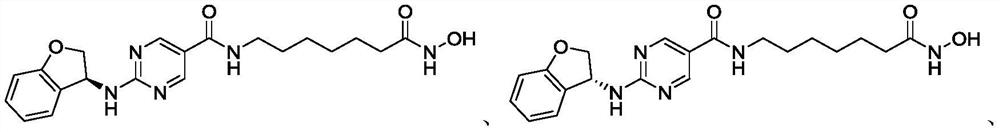
[0194] Example 3: N-(7-(hydroxylamino)-7-oxoheptyl)-2-(isochroman-4-ylamino)pyrimidine-5-carboxamide
[0195]
[0196] Step 1: Synthesis of 1-((allyloxy)methyl)-2-iodobenzene
[0197] Sodium hydride (60%, 1.15g, 28.8mmol) was added to tetrahydrofuran (100ml), and (2-iodophenyl)methanol (4.5g, 19.2mmol) was added at 0°C, and the reaction mixture was stirred at 0°C for half After hours 3-bromoprop-1-ene (2.53 g, 21.1 mmol) was added. The reaction mixture was stirred at 60°C for 16 hours. The reaction mixture was cooled to room temperature. Water (50ml) was added to the reaction mixture at 0°C, and extracted with ethyl acetate (100ml×3). The combined organic phases were washed with saturated brine (50 ml), dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain a crude product. Separation and purification by column chromatography (silica gel; petroleum ether: ethyl acetate = 20:1-5:1) gave the target compound (4.50 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com