Camptothecin derivatives and preparation method and application thereof
A kind of use and drug technology, applied in the field of medicine, can solve the problems of low selectivity, blocking replication and transcription, and large toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 120
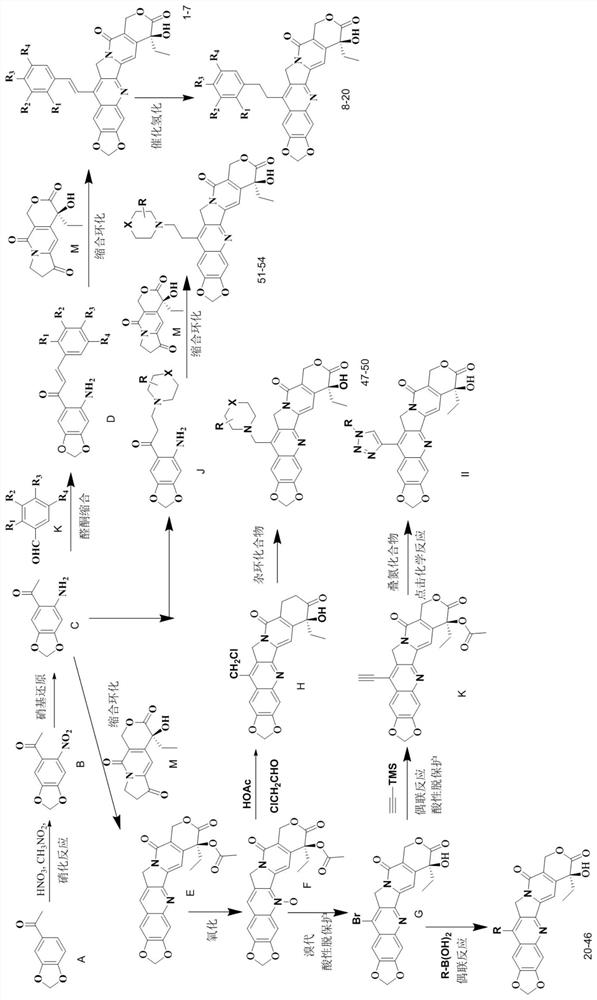
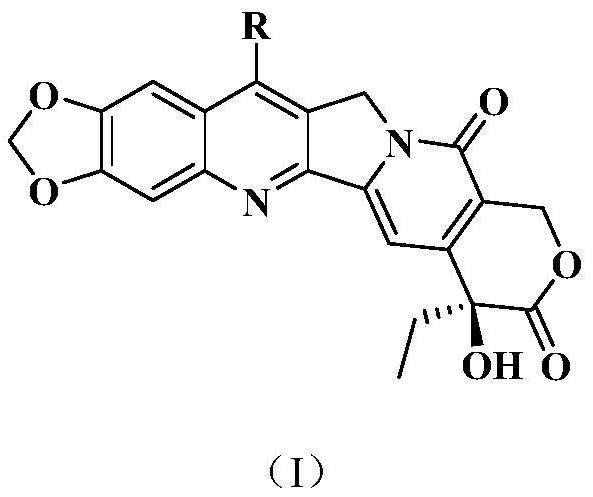
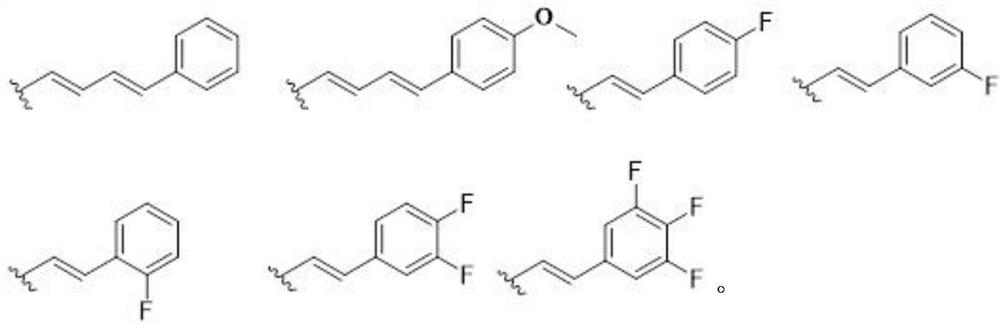
[0074] Example 1 Preparation of 20(S)-7-phenylbutadienyl-10,11-methylenedioxycamptothecin (compound 1)
[0075] Step (1) (2E,4E)-1-(5-Aminobenzo[d][1,3]dioxo-6-yl)-5-phenylpent-2,4-diene-1- Preparation of ketone (D1)
[0076] At room temperature, concentrated nitric acid (20.7ml) was slowly added dropwise to a solution of 3,4-methylenedioxyacetophenone (8.1g, 49.3mmol) in nitromethane (80ml), and the reaction was stirred for 2h. Slowly add saturated sodium bicarbonate solution dropwise, adjust the pH to neutral and extract three times with dichloromethane, combine the organic phases, wash three times with saturated brine, dry over anhydrous magnesium sulfate and concentrate to obtain a yellow oily liquid, which is subjected to silica gel column chromatography (petroleum ether:ethyl acetate=8:1) for separation and concentration to obtain 7.8 g of light yellow solid 6-nitro-3,4-methylenedioxyacetophenone, yield 76%; mp 110-112°C.
[0077] 6-Nitro-3,4-methylenedioxyacetophenone...
Embodiment 2
[0084] Example 2 Preparation of 20(S)-7-(4-fluorostyryl)-10,11-methylenedioxycamptothecin (compound 3)
[0085] Substitute 4-fluorobenzaldehyde for the cinnamaldehyde in step (1) of Example 1, and the other required raw materials, reagents, and preparation methods are the same as steps (1) and (2) of Example 1, to prepare 20(S)-7 -(4-Fluorostyryl)-10,11-methylenedioxycamptothecin yellow solid compound 3. mp>250℃; MS(ESI): m / z, 513.5[M+H] + .
[0086] 1 H NMR (500MHz, CF 3 COOD)δ8.23(s,1H),8.00(s,1H),7.95(dd,J=7.8,5.4Hz,1H),7.87(d,J=16.5Hz,1H),7.85–7.78(m, 1H), 7.73(s, 1H), 7.30(dd, J=22.8, 14.3Hz, 2H), 7.10(dd, J=36.5, 25.0Hz, 1H), 6.52(s, 2H), 6.03(t, J = 11.9Hz, 1H), 6.01–5.83 (m, 2H), 5.69 (d, J=17.0Hz, 1H), 2.24 (dd, J=17.8, 11.0Hz, 2H), 1.33–1.16 (m, 3H) .
[0087] 13 C NMR (125MHz, CF 3 COOD) δ176.2, 157.4, 152.1, 151.8, 148.9, 145.0, 140.1, 139.8, 138.8, 130.6, 130.0, 130.0, 126.1, 126.0, 121.0, 117.3, 116.1, 116.0, 105.0, 103.4, 10 52.5, 31.0, 11.7, 5.8.
Embodiment 3
[0088] Example 3 Preparation of 20(S)-7-(3-fluorostyryl)-10,11-methylenedioxycamptothecin (compound 4)
[0089] Substitute 3-fluorobenzaldehyde for the cinnamaldehyde in step (1) of Example 1, and the rest of the required raw materials, reagents, and preparation methods are the same as steps (1) and (2) of Example 1, to prepare 20(S)-7 -(4-Fluorostyryl)-10,11-methylenedioxycamptothecin yellow solid compound 4. mp>250℃; MS(ESI): m / z, 513.5[M+H] + .
[0090] 1 H NMR (500MHz, CF 3 COOD)δ8.23(s,1H),8.00(s,1H),7.95(dd,J=7.8,5.4Hz,1H),7.87(d,J=16.5Hz,1H),7.85–7.78(m, 1H), 7.73(s, 1H), 7.30(dd, J=22.8, 14.3Hz, 2H), 7.14(dd, J=36.5, 25.0Hz, 1H), 6.56(s, 2H), 6.03(t, J = 11.9Hz, 1H), 6.21–5.84 (m, 2H), 5.69 (d, J=17.0Hz, 1H), 2.24 (dd, J=17.8, 11.0Hz, 2H), 1.33–1.16 (m, 3H) .
[0091] 13 C NMR (126MHz, CF 3 COOD) δ176.2, 157.4, 152.1, 151.8, 148.9, 145.0, 140.1, 139.8, 138.8, 130.6, 130.4, 130.0, 126.8, 126.0, 121.0, 117.5, 116.1, 116.0, 105.0, 16.1, 3.9, 10 52.5, 31.0, 11.7,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com