Synthesis method and application of sulfonamide dimethylpyrimidine molecularly imprinted polymer
A technology of sulfamethazine and dimethylpyrimidine is applied in the field of synthesis of sulfonamide dimethylpyrimidine molecularly imprinted polymers, and can solve the problems of complex preparation process, many preparation process variables, general specificity and the like of surface imprinting technology , to achieve the effect of good identification and adsorption capacity, optimized solvent usage ratio, and excellent specific adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Study on the Specificity of Sulphonamide Molecularly Imprinted Polymers
[0050] 1. Preparation method:
[0051] (1) Preparation of MIP
[0052]According to template molecule: functional monomer: crosslinking agent: initiator, the addition ratio is 1mmol: 4mmol: 20mmol: 120mg to weigh each substance, that is, the amount of each substance is specifically: template molecule 0.27g sulfamethazine, 0.17g formazan Acrylic acid and 0.19g of diallylamine were dissolved in 4mL of methanol solvent, and pre-polymerized for 30min. After pre-polymerization, add 4mL cross-linking agent DVB and 120mg azobisisobutyronitrile initiator, sonicate for 10min, deoxygenate with nitrogen for 10min, seal and polymerize in a constant temperature water bath at 60°C for 12h to obtain a white solid.
[0053] After cooling the above reaction solution to room temperature, centrifuge, take the white precipitate, dry it, extract it with a mixed solvent of methanol and acetic acid with a vol...
Embodiment 2
[0060] Example 2 Optimization of functional monomer ratio
[0061] Based on the preparation and analysis method of MIP described in Example 1, under the same other conditions, a series of molecularly imprinted polymers were produced by changing the types of functional monomers, and the appropriate functional monomer types were screened on the basis of imprinting factors. The results showed that, Mixed functional monomers utilizing methacrylic acid and diallylamine are optimal.
[0062] Based on the preparation and analysis method of MIP described in Example 1, under the same other conditions, the ratio of the mixed functional monomer methacrylic acid and diallylamine was changed, wherein the designed molar ratio of the two was 4:0 in sequence , 3:1, 1:1, 1:3 and 0:4, the imprinting factors of MIP with different functional monomer ratios are shown in Table 1:
[0063] Table 1 Comparison table of specificity of MIP with different functional monomer ratios
[0064]
[0065] ...
Embodiment 3
[0067] Example 3 Cross-linking agent optimization
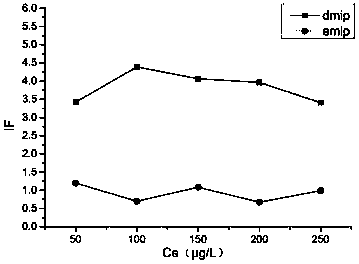
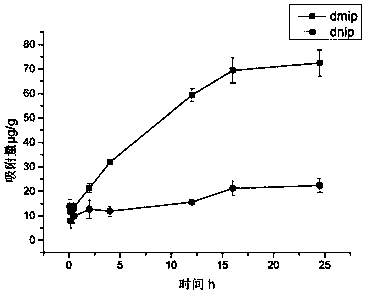
[0068] Based on the experimental group 2-3 in Example 2, under the same other conditions, molecularly imprinted polymer MIPs with different crosslinking agents were prepared. The MIP and NIP prepared by the linking agent were named eMIP and eNIP, respectively, and the MIP and NIP prepared by DVB as the crosslinking agent were named dMIP and dNIP. The morphology and structure of eMIP and dMIP are as follows: figure 1 As shown, the IF values of the two molecularly imprinted polymers in different concentrations of sample solutions are as follows figure 2 shown.
[0069] combine figure 1 and figure 2 It can be seen that the molecularly imprinted polymers prepared by different cross-linking agents have different shapes and specificities. The dMIP obtained by DVB is spherical, and the specific surface area is smaller than that of eMIP obtained by EGDMA, but the specificity is stronger than eMIP. In the methylpyrimidine sta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com