Novel application of 2,3,5,4-tetrahydroxy-stilbene-2-O-beta-D-Glucoside (TSG) in preventing and/or treating hyperhomocysteinemia (HHcy)
A technology of cysteinemia and stilbene glycosides, which is applied in the field of medicine, can solve problems that have not been reported before, achieve wide application prospects, inhibit the upregulation of B-type endothelin receptors, and inhibit the activation of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
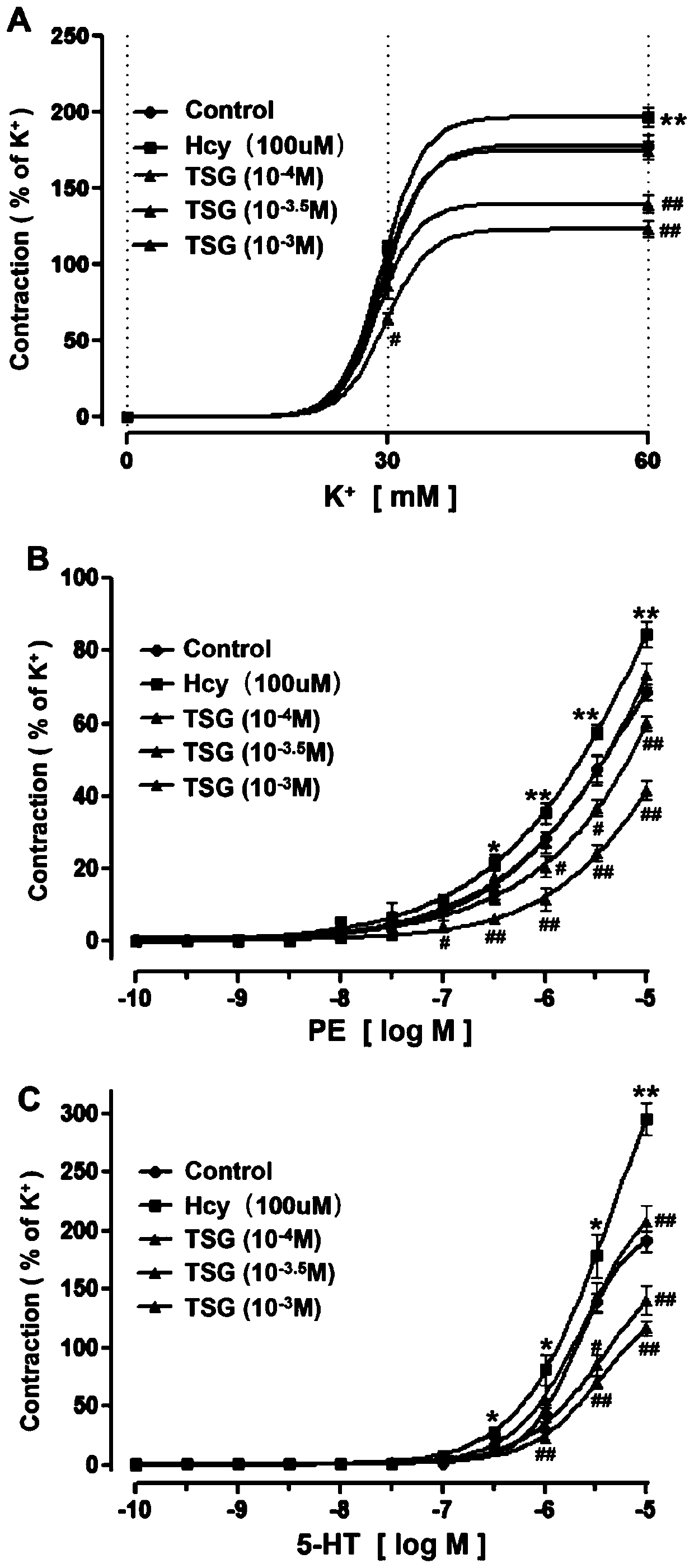
[0012] Example 1. TSG inhibits Hcy-induced vascular smooth muscle contraction response
[0013] Different concentrations of TSG (10 -4 M, 10 -3.5 M, 10 -3 M) respectively co-incubated with Hcy (100 μM) for 24 hours to observe the effect of TSG on Hcy-induced vasoconstriction induced by vasoconstrictor substances (KCl, PE or 5-HT) in rat superior mesenteric artery. The results showed that, compared with the Hcy (100μM) group, TSG (10 -3.5 M, 10 -3 M) It can inhibit the vasoconstriction response of blood vessels induced by Hcy to KCl, PE or 5-HT (Pfigure 1 A, B, C). These results show that TSG can reduce the sensitivity of Hcy-induced blood vessels to vasoconstrictor substances, and inhibit the constriction effect of such substances on blood vessels.
Embodiment 2
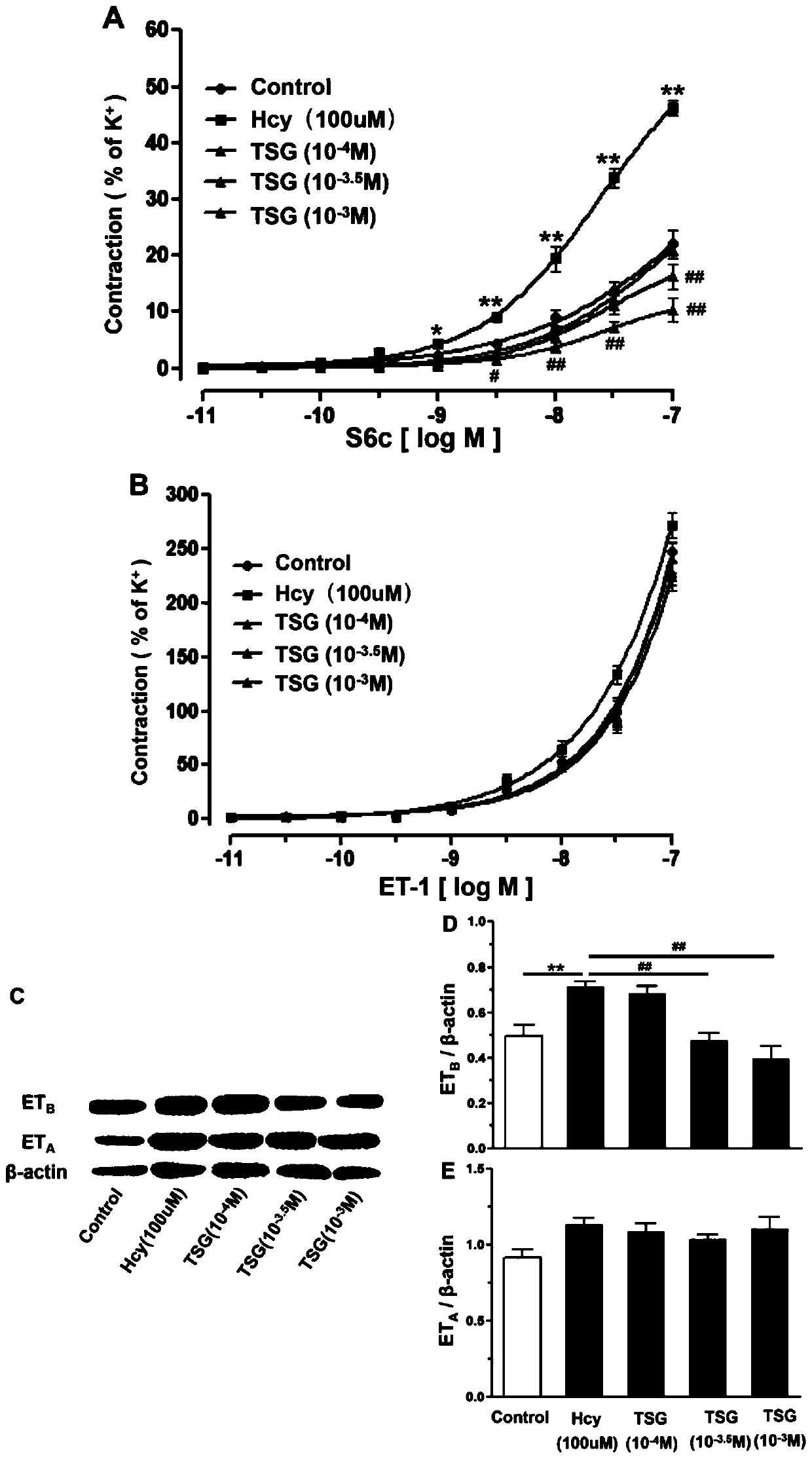
[0014] Example 2. TSG inhibits the endothelin receptor-mediated contraction of vascular smooth muscle and the expression of its receptor protein caused by Hcy. Different concentrations of TSG (10 -4 M, 10 -3.5 M, 10 -3 M) Co-incubated with Hcy (100 μM) for 24 hours, and observed the ET of TSG on Hcy A and ET B Receptor-Mediated Contraction Response and ET in Rat Superior Mesenteric Artery A and ET B Effect of receptor protein expression. The results showed that, compared with the Hcy (100μM) group, TSG (10 -3.5 M, 10 -3 M) It can inhibit the vasoconstriction response induced by ET-1 or S6c induced by Hcy (P figure 2 A, B). Moreover, TSG significantly downregulated Hcy-increased superior mesenteric artery ET B Receptor level (P figure 2 C, D, E). These results show that TSG can downregulate Hcy-induced ET B Receptor upregulation and its mediated vasoconstrictor response.
Embodiment 3
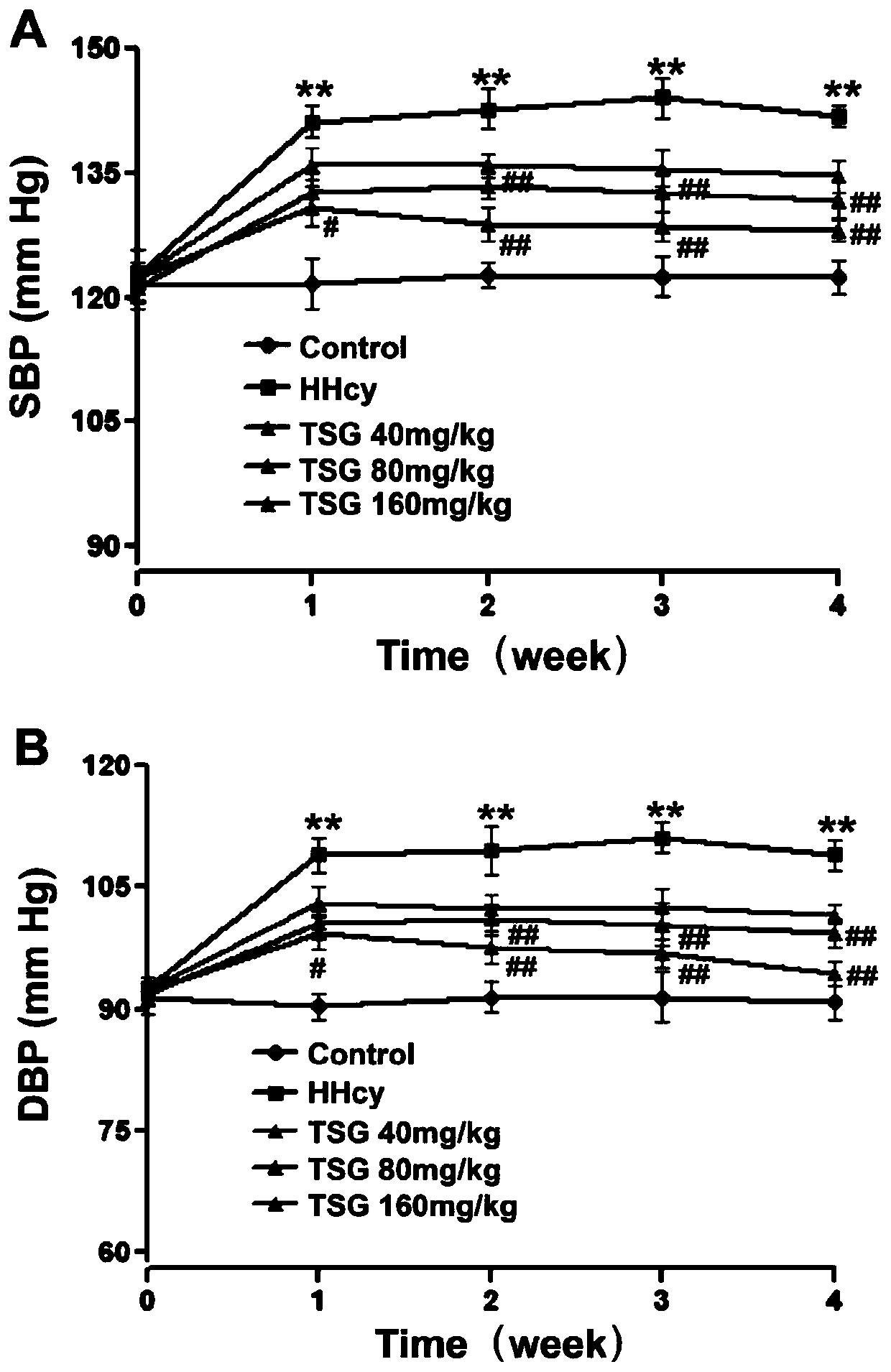
[0015] Example 3. Effect of TSG on blood pressure of HHcy mice
[0016] The systolic blood pressure and diastolic blood pressure of the mice in the HHcy group were significantly higher than those in the control group in the first, second, third, and fourth weeks of the experiment (P image 3 A, B). These data suggest that TSG can inhibit the HHcy-induced blood pressure increase.
[0017] In summary, the experimental studies have confirmed that stilbene glycoside has a therapeutic effect on hyperhomocysteinemia in experimental animals, suggesting that it has a significant prospect of being used in the development of prevention and treatment of hyperhomocysteinemia.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com