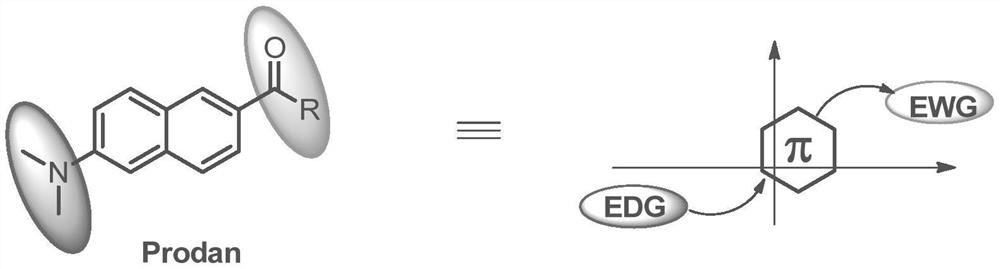
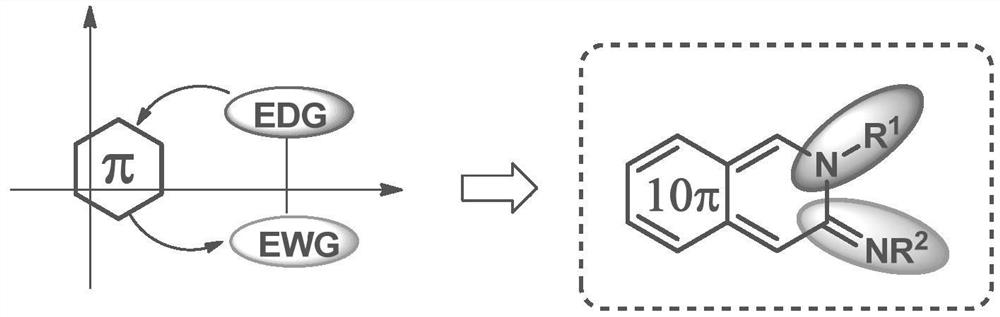
A cyclic amidinyl fluorescent molecule with the same-side push-pull electron effect and its preparation method
A technology of fluorescent molecules and push-pull electrons, applied in the field of biochemical detection, can solve problems such as poor signal-to-noise ratio, limited application, limited sensitivity, etc., and achieves the effects of mild conditions, simple preparation process, and stable fluorescent properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] This embodiment provides a cyclic amidinyl fluorescent molecule with the same-side push-pull electron effect, and its structural formula is: Among them, R 1 Hydrogen, fluorine, chlorine, bromine, iodine, methoxyl, hydroxyl, diethylamine, piperidine, tetrahydropyrrole, nitro, aryl or cyano connected to the 5, 6, 7, 8 positions of the 10π electron ring A kind of R 2 A five-membered aromatic ring with no substituents or 1-4 substituents or a six-membered aromatic ring with no substituents or 1-4 substituents, a nitrogen-containing heterocycle, an oxygen-containing heterocycle or a sulfur-containing heterocycle , the R 3 p-toluenesulfonyl, methanesulfonyl, alkyl, unsubstituted or a five-membered aromatic ring with 1-4 substituents, or a six-membered aromatic ring with no substituent or 1-4 substituents, nitrogen-containing Heterocycle, oxygen-containing heterocycle or sulfur-containing heterocycle.
[0041] The fluorescent molecule is obtained by reacting differently sub...
Embodiment 2
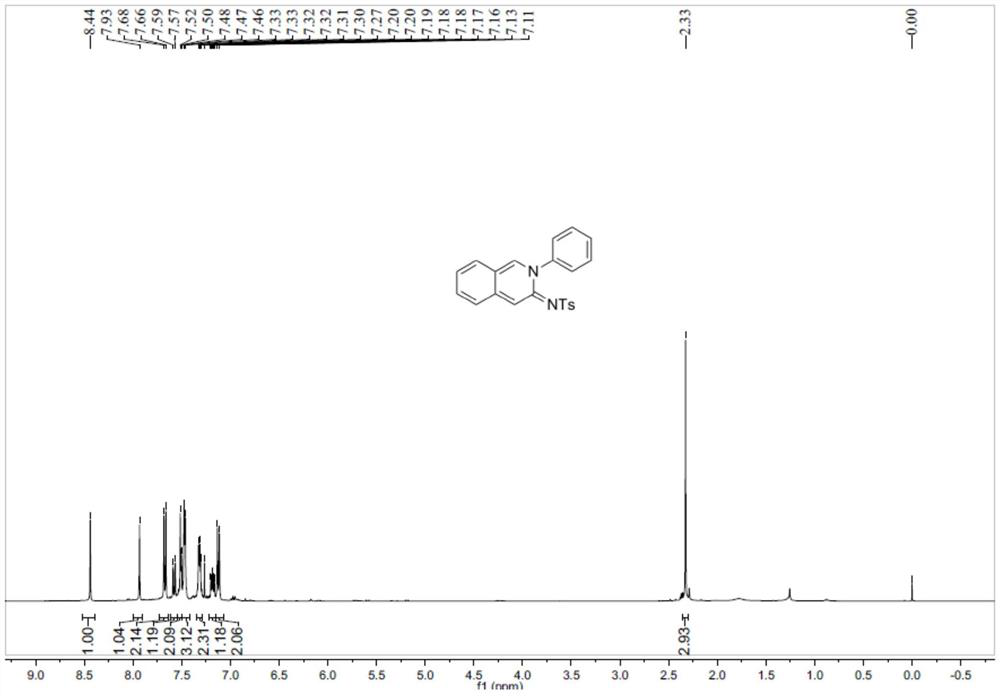
[0057] This embodiment provides a cyclic amidinyl fluorescent molecule with the same-side push-pull electron effect, and its structural formula is: It is prepared from N-sulfonyltriazolebenzaldehyde and o-toluidine, and its preparation method is basically the same as that of Example 1.
[0058] The NMR spectrogram of the cyclic amidinyl fluorescent molecule described in the present embodiment is as follows Figure 8-9 As shown, the test data is as follows:
[0059] 1 H NMR (400MHz, CDCl 3 ):δ8.34(s,1H),8.02(s,1H),7.67(d,J=8.2Hz,2H),7.63–7.48(m,3H),7.44(dd,J=10.8,4.1Hz, 1H), 7.41–7.32(m,2H), 7.25–7.08(m,4H), 2.34(s,3H), 2.02(s,3H).
[0060] 13 C NMR (100MHz, CDCl 3 ): δ152.6, 142.0, 142.0, 141.5, 141.2, 141.0, 134.3, 134.0, 131.2, 129.9, 128.8, 127.7, 127.2, 126.4, 126.2, 125.9, 125.3, 120.0, 111.5, 21.3, 17.6
[0061] In the infrared (IR) spectrum of the fluorescent molecular product, the positions of the absorption peaks are respectively located at: 2922, 1640, 1600, ...
Embodiment 3
[0065] This embodiment provides a cyclic amidinyl fluorescent molecule with the same-side push-pull electron effect, and its structural formula is: It consists of N-sulfonyltriazole benzaldehyde and Prepared, its preparation method is basically the same as that of Example 1.
[0066] The NMR spectrogram of the cyclic amidinyl fluorescent molecule described in the present embodiment is as follows Figure 11-12 As shown, the test data is as follows:
[0067] 1 H NMR (400MHz, CDCl 3 ): δ8.45(s,1H),7.95(s,1H),7.74(d,J=8.2Hz,2H),7.59(d,J=8.6Hz,1H),7.52(d,J=3.4Hz ,2H),7.38(dt,J=11.8,4.1Hz,6H),7.25–7.18(m,1H),7.11(ddd,J=9.8,8.4,4.9Hz,3H),7.00(t,J=2.2 Hz, 1H), 6.92 (dd, J=7.8, 1.2Hz, 1H), 4.97 (s, 2H), 2.36–2.26 (m, 3H).
[0068] 13 C NMR (100MHz, CDCl 3 ): δ159.3, 152.4, 142.6, 141.8, 141.5, 141.1, 136.3, 134.1, 130.2, 128.9 128.7, 128.2, 127.9, 127.6, 126.2, 125.8, 125.3, 119.9119.0, 116.4, 110.13.1
[0069] In the infrared (IR) spectrogram of the fluorescent molecular pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com