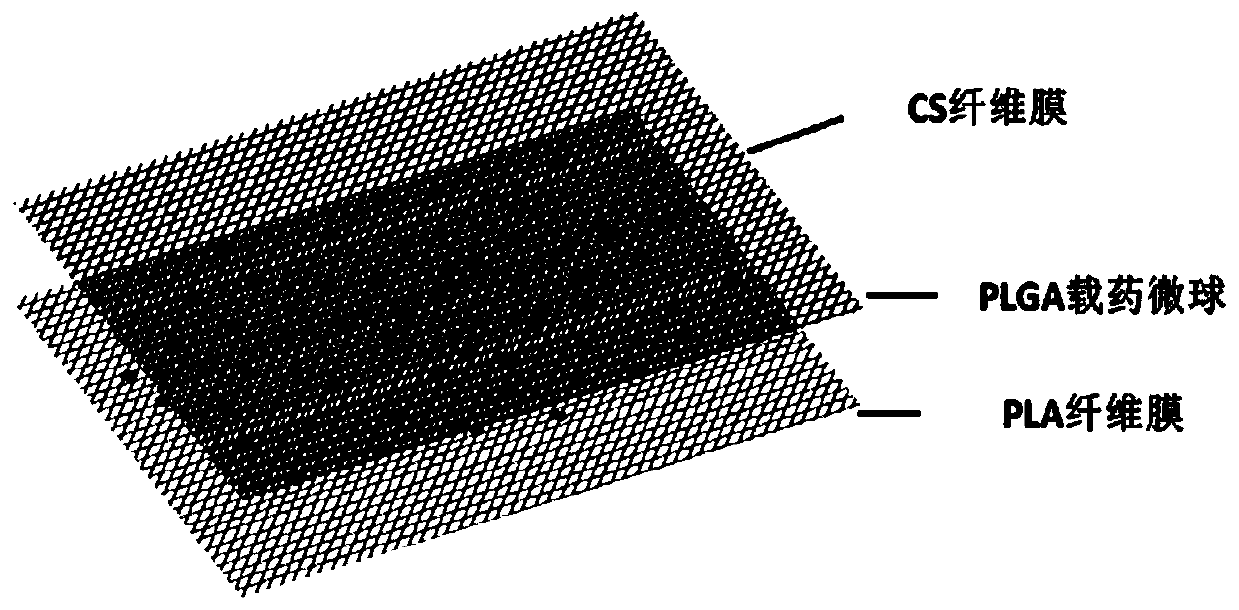
Composite membrane carrying bioactive factors PLA/PLGA/CS and preparation method thereof
A bioactive factor, composite membrane technology, used in tissue regeneration, medical science, prosthesis, etc., can solve the problems of difficult to control the direction and rate of drug release, unable to meet the time required for spinal cord repair, and irregular spinal cord injury. Achieve the effect of prolonging drug release time, helping nerve repair, and good drug release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
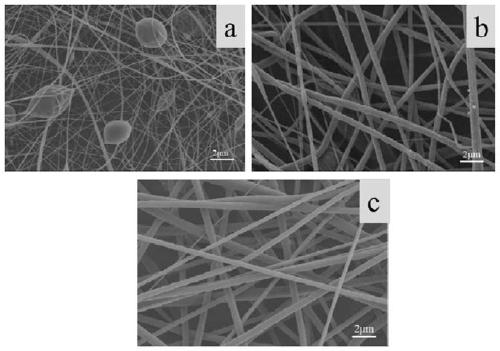
[0038] Example 1: Preparation of PLA fiber membrane.
[0039] (1) dissolving PLA in hexafluoroisopropanol to form PLA solutions of 6wt%, 7wt% and 8wt% respectively;
[0040] (2) Put different concentrations of PLA solutions into plastic syringes with a capacity of 10ml, place the syringes horizontally on the syringe pump, cover a metal plate with a layer of aluminum foil as a receiver, and use a 20-gauge needle for the nozzle of the syringe. Electrospinning conditions: voltage 13-18kV, flow rate 0.08-0.1mm / min, receiving distance 12-15cm;
[0041] (3) The fiber membrane obtained in (2) is removed from the aluminum foil and freeze-dried to obtain a PLA fiber membrane.
Embodiment 2
[0042] Example 2: Preparation of PLGA microspheres.
[0043] (1) first dissolve PLGA in chloroform to be made into 5wt%, 6wt% and 7wt% PLGA solution, stir magnetically until completely dissolved;
[0044] (2) Electrospray PLGA solutions with different concentrations, put the electrospray solution into a plastic syringe with a capacity of 10ml, place the syringe horizontally on the syringe pump, and cover a metal plate with a layer of aluminum foil as a receiver. The nozzle of the syringe is a No. 22 needle. EFI conditions: Voltage 8-12kV, flow rate 0.06-0.08mm / min, receiving distance 12-15cm. Obtain PLGA microspheres.
Embodiment 3
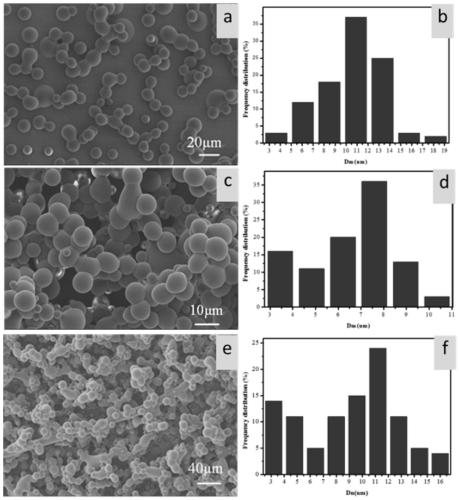
[0045] Example 3: Preparation of drug-loaded microspheres with a chloroform solution concentration of PLGA of 6 wt % and water-to-oil ratios of 1:50, 1:100 and 1:200, respectively.
[0046] (1) PLGA is dissolved in chloroform, and magnetically stirred to completely dissolve, obtains the PLGA organic phase solution of 6wt%;
[0047] (2) An aqueous phase solution was prepared by dissolving NGF in deionized water. Then, the aqueous phase solution and the PLGA organic phase solution were mixed and sonicated (5 cycles, 10 seconds, 300W) to form emulsions with water-to-oil ratios of 1:50, 1:100, and 1:200, with a drug content of 0.5 μg / ml;
[0048] (3) Electrospray emulsions with different water-to-oil ratios, put the electrospray liquid into a plastic syringe with a capacity of 10ml, place the syringe horizontally on the syringe pump, and cover a metal plate with a layer of aluminum foil as a receiver , The syringe nozzle selects 22 gauge needles. EFI conditions: Voltage 8-12kV...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com