Process for preparing 2-cyan-4-nitroaniline through micro-channel reaction
A micro-channel reaction and micro-channel reactor technology, which is applied in the preparation of carboxylic acid nitrile, the preparation of organic compounds, chemical instruments and methods, etc. problem, to achieve the effect of short reaction time, small amount of ammonia and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
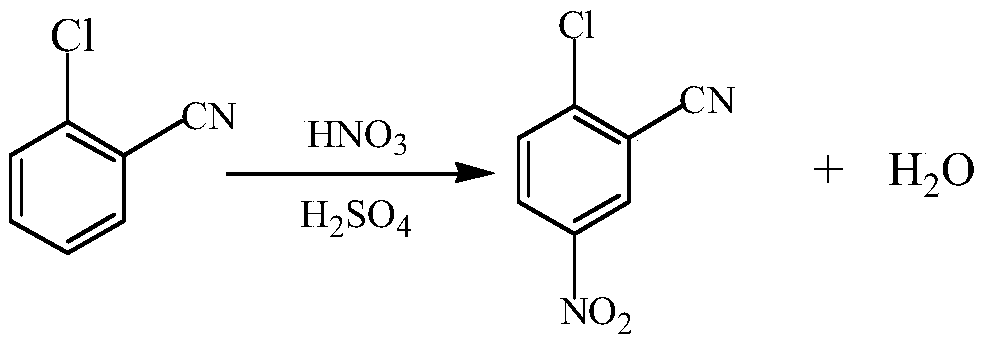
[0026] Dissolve 100kg of o-chlorobenzonitrile in 200kg of dichloroethane, prepare evenly as material 1, prepare evenly 55kg of concentrated nitric acid and 100kg of concentrated sulfuric acid as material 2, set the microchannel reaction temperature to 25°C, start feeding, and The flow rate of material 1 is 2000ml / min, the flow rate of material 2 is 1280ml / min, the pressure in the microchannel is 2.0-2.9MPa, and the reaction is carried out for 10 minutes. The reaction mixture is subjected to water analysis, filtration, layering and other operations to obtain some intermediate 2-chloro-5- Nitrobenzonitrile, add water to the organic layer to distill to recover dichloroethane, and some intermediates dissolved in dichloroethane are dispersed in water, filtered, washed for later use, and the yield of nitration reaction is 94% according to statistics.
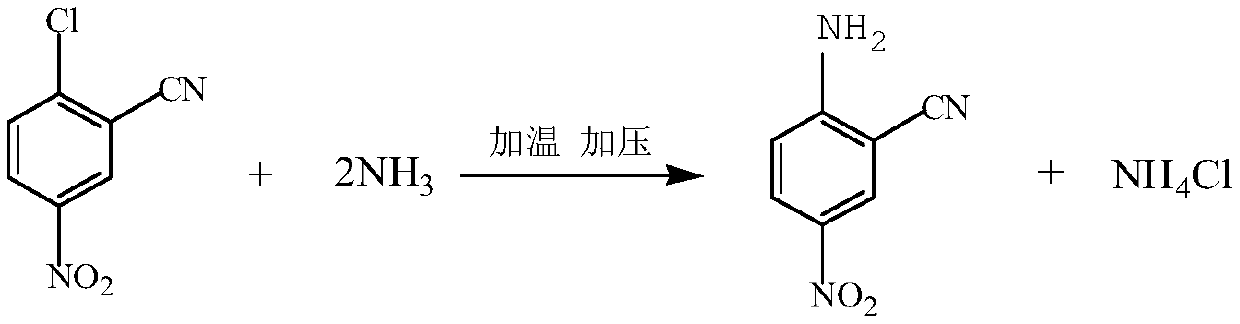
[0027] After mixing 60kg of the above-mentioned intermediate with 300kg of ethanol, transfer it to a 1000L high-pressure reactor, qua...
Embodiment 2
[0029] Dissolve 100kg of o-chlorobenzonitrile in 300kg of dichloroethane, prepare evenly as material 1, prepare evenly 55kg of concentrated nitric acid and 120kg of concentrated sulfuric acid as material 2, set the microchannel reaction temperature to 30°C, start feeding, and The flow rate of material 1 is 2200ml / min, the flow rate of material 2 is 1200ml / min, the pressure in the microchannel is 2.0-2.9MPa, and the reaction is carried out for 10 minutes. Nitrobenzonitrile, the organic layer was distilled with water to recover dichloroethane, and some intermediates dissolved in dichloroethane were dispersed in water, filtered, and washed for later use. According to statistics, the yield of nitration reaction is 92%.
[0030] After mixing 60kg of the above-mentioned intermediate with 300kg of methanol, transfer it to a 1000L high-pressure reactor, quantitatively feed 70kg of ammonia gas, the reaction temperature is 100°C, and the reaction time is 4 hours. The finished product w...
Embodiment 3
[0032] Dissolve 100kg of o-chlorobenzonitrile in 350kg of dichloroethane, prepare evenly as material 1, prepare evenly 55kg of concentrated nitric acid and 150kg of concentrated sulfuric acid as material 2, set the microchannel reaction temperature to 35°C, start feeding, and The flow rate of material 1 is 2500ml / min, the flow rate of material 2 is 1500ml / min, the pressure in the microchannel is 2.0-2.9MPa, and the reaction is carried out for 10 minutes. The reaction mixture is subjected to water analysis, filtration, layering and other operations to obtain some intermediate 2-chloro-5- Nitrobenzonitrile, the organic layer was distilled with water to recover dichloroethane, and some intermediates dissolved in dichloroethane were dispersed in water, filtered, and washed for later use. According to statistics, the yield of nitration reaction is 92%.
[0033] After mixing 60kg of the above-mentioned intermediate with 300kg of dichloroethane evenly, transfer it to a 1000L high-pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com