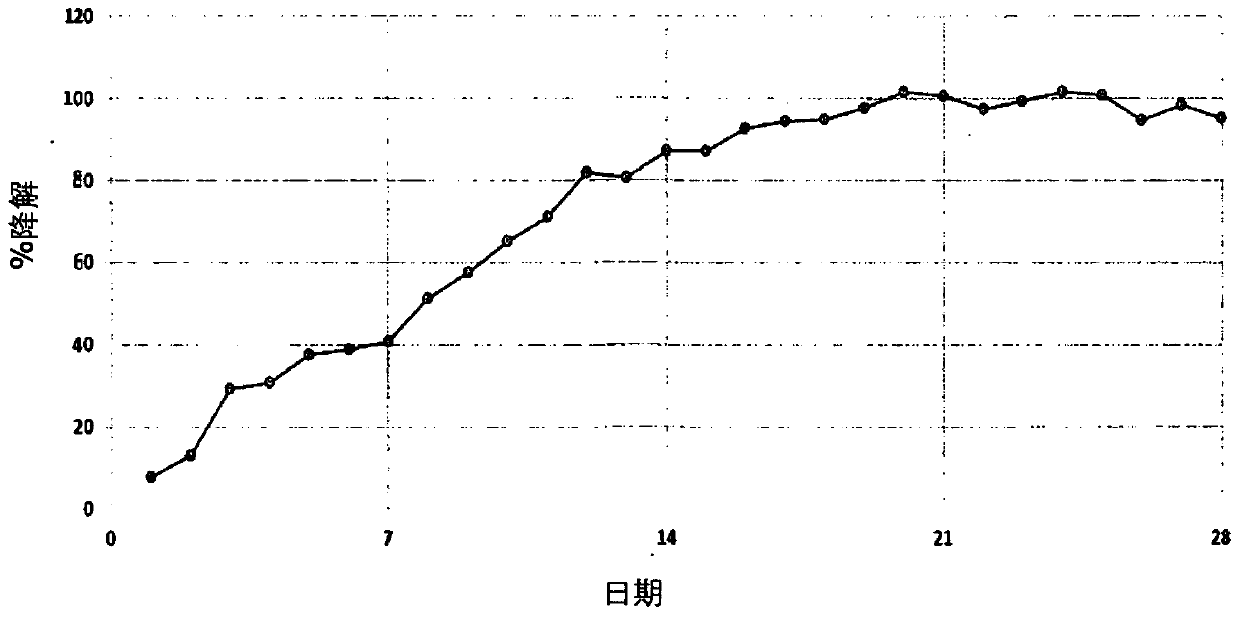
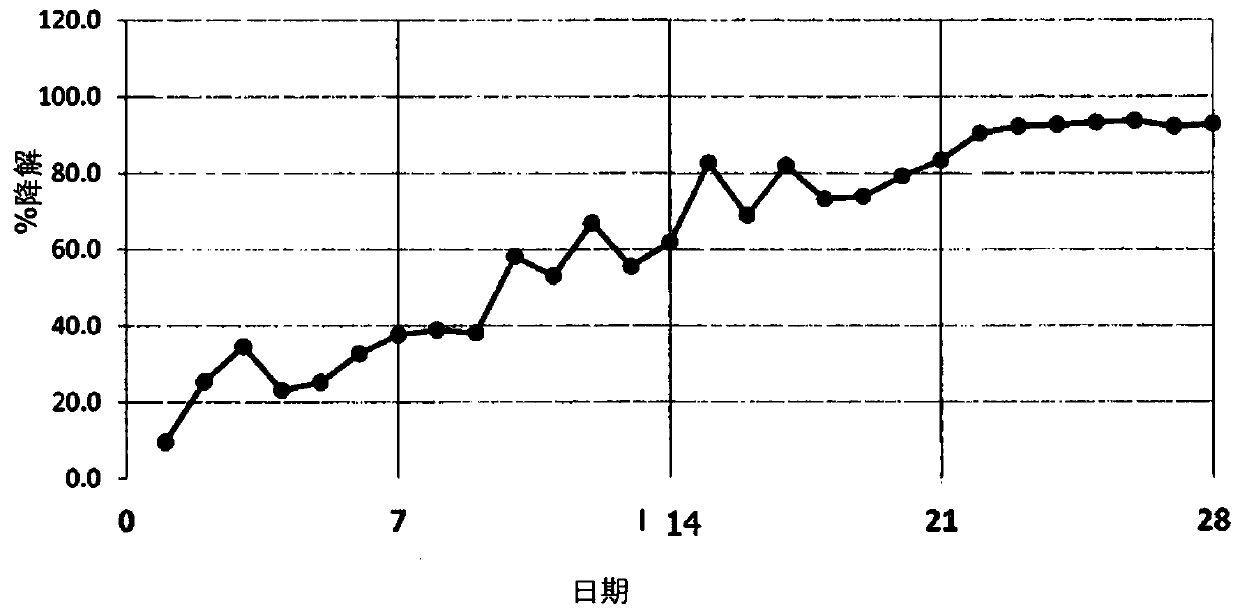
Biodegradable diethanolamine derivative chelating agent and preparation process thereof
A technology of diethanolamine and chelating agent, applied in the field of biodegradable diethanolamine derivative chelating agent and preparation thereof, can solve the problems of difficult removal and expensive transition metal catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Preparation of diethanolamine derivatives from reaction with maleic anhydride (ligand 1)
[0055]
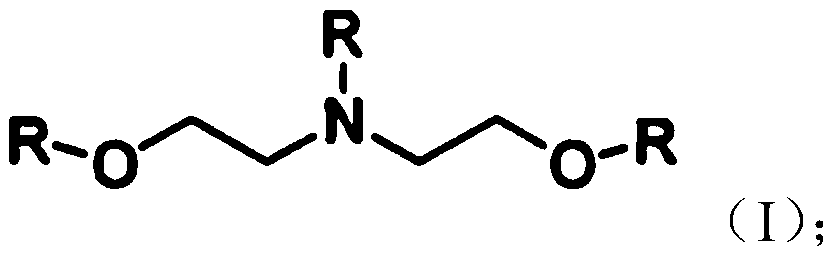
[0056] Diethanolamine derivatives and maleic anhydride can be synthesized according to the reaction of formula (I). The diethanolamine precursor, maleic anhydride, and selected catalysts were added to approximately 15-20 mL of dichloromethane solvent, with the amounts of each compound indicated in Table 1. Then, the resulting mixture was refluxed at 50 °C until the reaction was complete. Monitor the amount of precursor used. The solvent was removed from the obtained product. The obtained product was dissolved in 50 mL of distilled water, and washed at least 3 times with dichloromethane (30 mL each time). After washing, the obtained product was dried under vacuum and used 1 H NMR spectroscopy and liquid chromatography-mass spectrometry (LC / MS) techniques were used to analyze its properties. The % yields and calcium chelation values of the synthesized...
Embodiment 2
[0064] Example 2: Preparation of diethanolamine derivatives from reaction with succinic anhydride (ligand 2)
[0065]
[0066] Diethanolamine derivatives and succinic anhydride can be synthesized by reaction according to formula (II). The diethanolamine precursor, succinic anhydride, and selected catalysts were added to approximately 15–20 mL of dichloromethane solvent, with the amounts of each compound indicated in Table 3. Then, the resulting mixture was refluxed at 50 °C until the reaction was complete. Monitor the amount of precursor used. The solvent was removed from the obtained product. The obtained product was dissolved in distilled water (50 mL), and washed with dichloromethane at least 3 times (30 mL each). After washing, the obtained product was dried under vacuum and used 1 H NMR spectroscopy and liquid chromatography-mass spectrometry (LC / MS) techniques were used to analyze its properties. The % yields and calcium chelation values of the synthesized di...
Embodiment 3
[0074] Example 3: Preparation of diethanolamine derivatives from reaction with phthalic anhydride (ligand 3)
[0075]
[0076] Diethanolamine derivatives and phthalic anhydride can be synthesized by a reaction according to formula (III). Add the diethanolamine precursor, phthalic anhydride, and the selected catalyst to about 15-20 mL of dichloromethane solvent, where the amount of each compound is shown in Table 5. Then, the resulting mixture was refluxed at 50 °C until the reaction was complete. Monitor the amount of precursor used. The solvent was removed from the obtained product. The obtained product was dissolved in distilled water (50 mL), and washed with dichloromethane at least 3 times (30 mL each). After washing, the obtained product was dried under vacuum and used 1 H NMR spectroscopy and liquid chromatography-mass spectrometry (LC / MS) techniques were used to analyze its properties. The % yields and calcium chelation values of the synthesized diethanolami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com