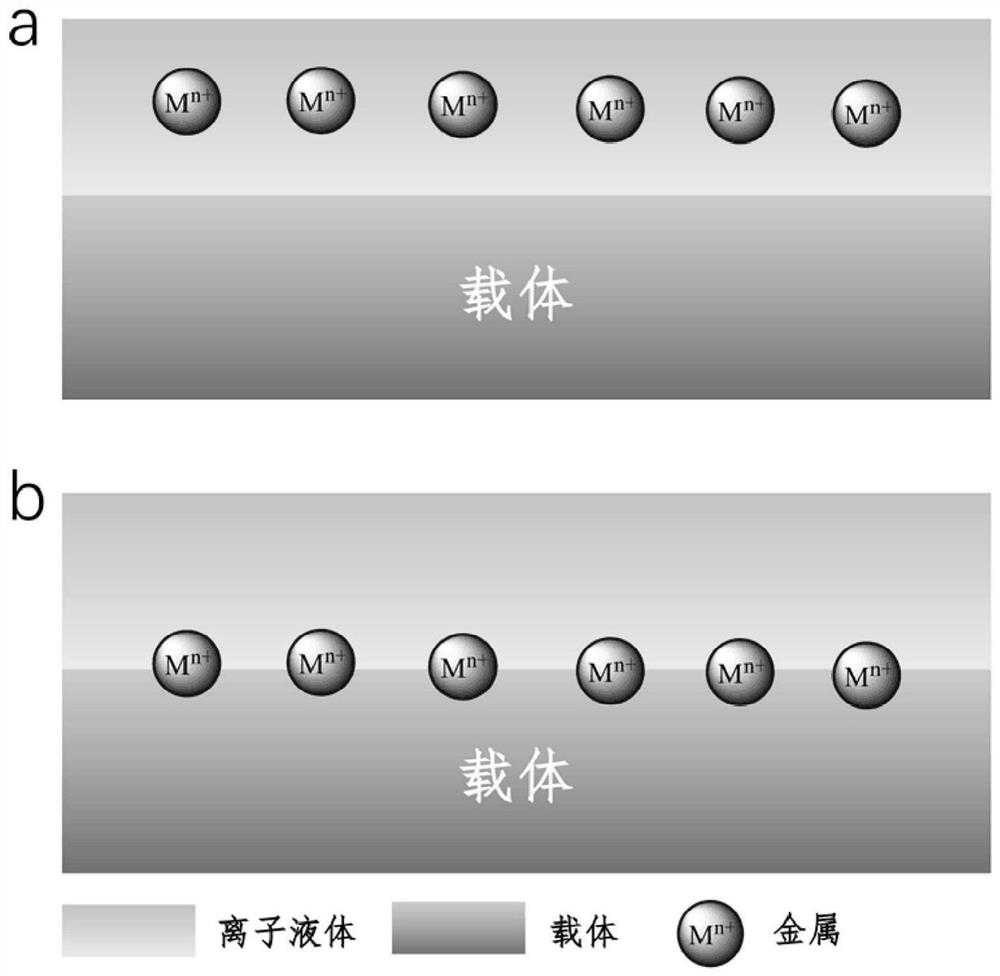
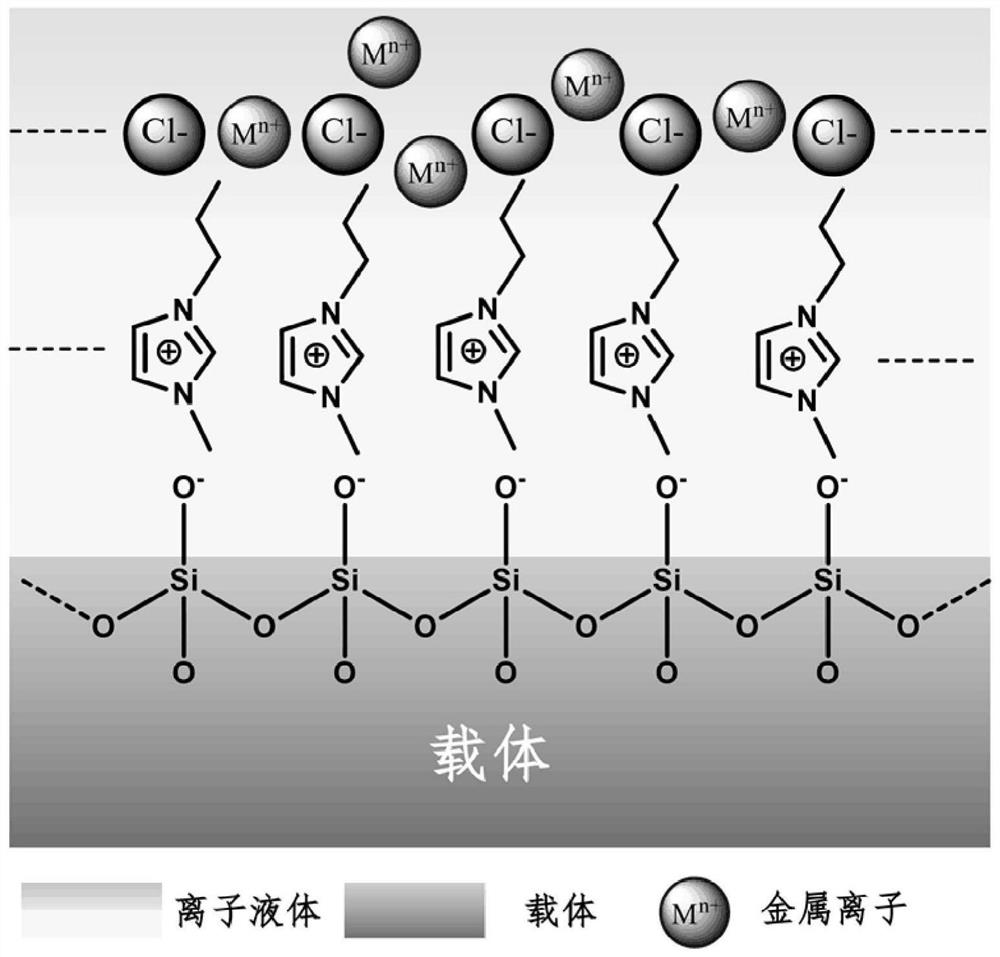
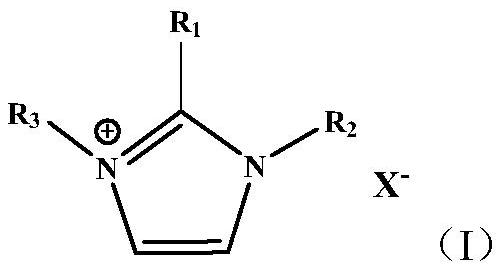
A kind of supported ionic liquid catalyst and its preparation method and application
An ionic liquid and catalyst technology, which is applied to the supported ionic liquid catalyst and its preparation and application fields, can solve the problems of low mass transfer and low gas metal dispersibility, and achieve the effects of high catalytic activity, high stability and not easy to run off.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Catalyst preparation:
[0077] 1) Select 10 g of silicon carbide as the carrier, dissolve it in 40 mL of toluene, stir for 30 min, and then add 0.1 g of dimethyldichlorosilane and 0.1 g of triethylamine. The above mixture was heat-treated under nitrogen at 50° C. for 2 hours. After filtering, the filter cake was washed with toluene and ethanol, dried at 180°C, and the obtained solid sample was used for future use.
[0078] 2) Take 0.05g of 1-propyl-2,3-dimethylimidazole bis-trifluoromethanesulfonimide salt and 0.05g of 1-hexyl-2,3-dimethylimidazole bis-trifluoromethanesulfonimide salt ion The liquid was dissolved in 0.5mL toluene, and after stirring evenly, the solid sample obtained in step 1) was added, soaked for 2 hours, and dried at 180°C for later use. Wherein the mass loading of ionic liquid is 1%.
[0079] 3) Dissolve 0.009g of chloroauric acid in 0.09mL of toluene, stir evenly, add the sample obtained in step 2), dip in an external 0.2kV static electric field...
Embodiment 2
[0084] Catalyst preparation:
[0085] 1) Select 10g of boron carbide as the carrier, dissolve it in 200mL of nitrogen-nitrogen dimethylformamide, stir for 30min, and then add 0.2g of dimethyldichlorosilane and 1g of triethylamine. The above mixture was heat-treated under nitrogen at 60° C. for 2.5 hours. After filtering, the filter cake was washed with toluene and ethanol, dried at 180°C, and the obtained solid sample was used for future use.
[0086] 2) Dissolve 0.4 g of 1-butyl-3-methylimidazolium hexafluorophosphate and 1.6 g of 1-propyl-3-butylimidazolium tetrafluoroborate ionic liquid in 10 mL of nitrogen-nitrogen dimethylformamide , after stirring evenly, add the solid sample obtained in step 1), soak for 3 hours, and dry at 180°C for use. Wherein the mass loading of ionic liquid is 20%.
[0087] 3) Dissolve 0.103g of ruthenium chloride in 0.5mL of nitrogen-nitrogen dimethylformamide, stir evenly, add the sample obtained in step 2), and immerse in an external 4kV stat...
Embodiment 3
[0092] Catalyst preparation:
[0093] 1) Select 10 g of titanium carbide as the carrier, dissolve it in 70 mL of azaalkylpyrrolidone, stir for 30 min, and then add 0.6 g of dimethyldichlorosilane and 0.2 g of triethylamine. The above mixture was heat-treated under nitrogen at 70° C. for 3 hours. After filtering, the filter cake was washed with toluene and ethanol, dried at 180°C, and the obtained solid sample was used for future use.
[0094] 2) Dissolve 0.15g of 1-butyl-3-methylimidazolium hexafluorophosphate and 0.35g of nitrogen-methylpyrrolidone hydrochloride ionic liquid in 50mL of nitrogen-methylpyrrolidone, stir evenly, and add the obtained in step 1) The solid samples were soaked for 4 hours and dried at 180°C for later use. Wherein the mass loading of ionic liquid is 5%.
[0095]3) Dissolve 4.23g of copper chloride in 2.12mL of nitrogen-methylpyrrolidone, stir evenly, add the sample obtained in step 2), dip in an external 3kV static electric field for 3h, and dry a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com