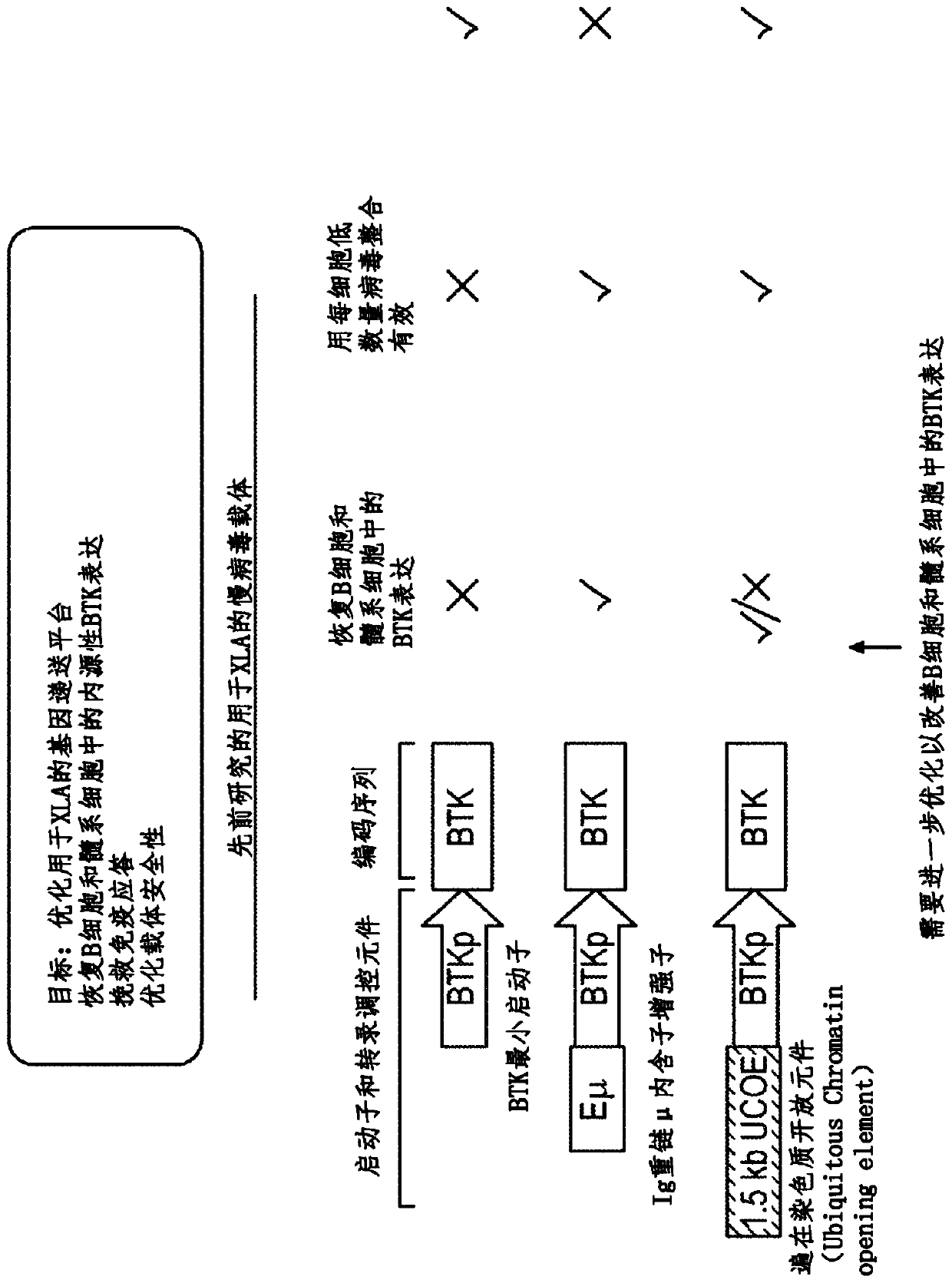
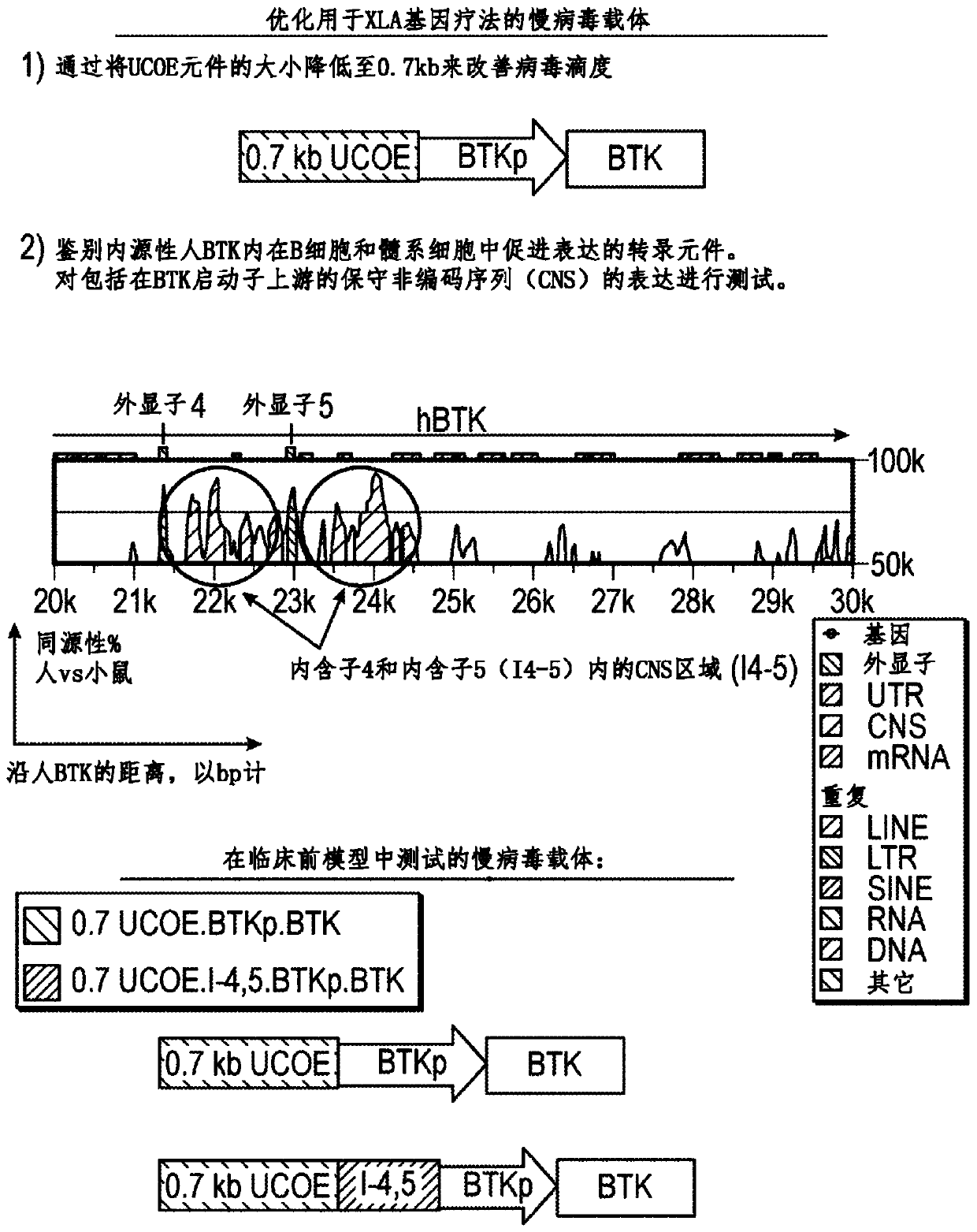
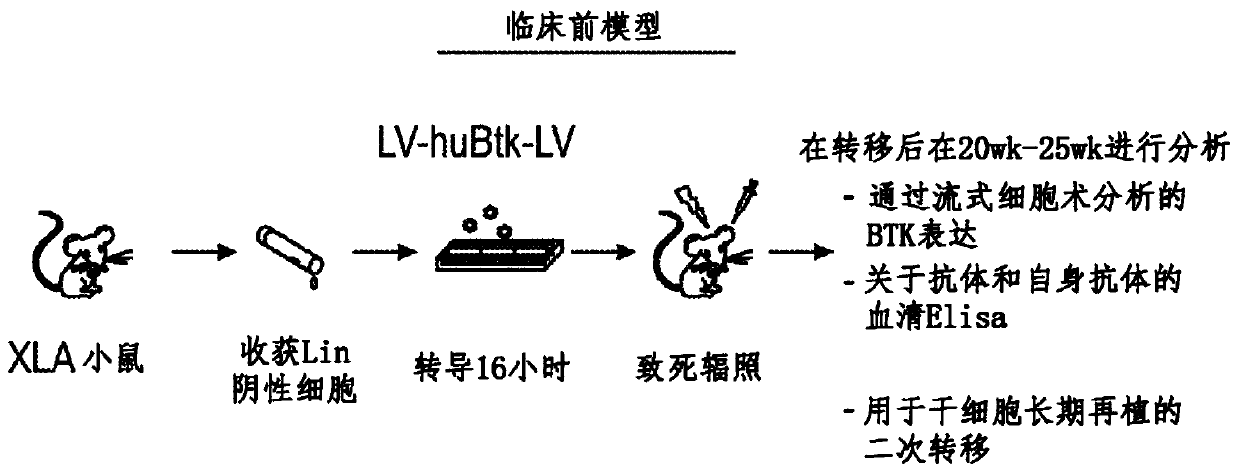
Optimized lentiviral vector for xla gene therapy
A carrier and polynucleotide technology, applied in gene therapy, medical raw materials derived from viruses/phages, viruses/phages, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0147] BTK is expressed on both B cells and myeloid cells, where BTK also contributes to normal functional responses in both lineages. Failure to express BTK results in XLA. Conversely, overexpression of activated BTK or wild-type BTK leads to cellular transformation and / or developmental arrest ("Early arrest in B cell development in transgenic mice that express the E41KBruton's tyrosine kinase mutant under the control of the CD19 promoterregion" J Immunol .1999Jun 1; 162(11):6526-33; "Correction of B-cell development in Btk-deficient mice using lentiviral vectors with codon-optimized humanBTK." Leukemia.2010Sep; 24(9):1617-30; cited incorporated herein in its entirety), and dysregulated expression of wild-type BTK can promote autoantibody production and increase the risk of autoimmunity (“Enhanced Expression of Bruton's Tyrosine Kinase in B Cells Drives Systemic Autoimmunity by Disrupting T Cell Homeostasis.” J Immunol. 2016 Jul 1; 197(1):58-67; incorporated herein by refere...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com