Catalyst for synthesizing methanol from carbon dioxide hydrogenation
A carbon dioxide and methanol synthesis technology, applied in the field of energy chemistry, can solve the problems of expensive metal Pd, difficult industrial process, sintering deactivation, etc., and achieve the effect of good environmental value, low production cost and easy control of conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] 1. 20ml aqueous solution of 0.73mmol ammonium molybdate and 10ml carbon disulfide are sealed in a 40ml autoclave under nitrogen protection;
[0019] 2. Keep the autoclave in (1) at 400°C for 4 hours to obtain a sample solution;
[0020] 3. (2) the obtained sample solution was treated in 30% (mass concentration) ammonia solution for 3 hours, then washed with water and ethanol for many times and suction filtration to obtain the sample;
[0021] 4. After drying the sample obtained in (3) at 100° C. for 12 hours, a sample of catalyst Cat1 was obtained.
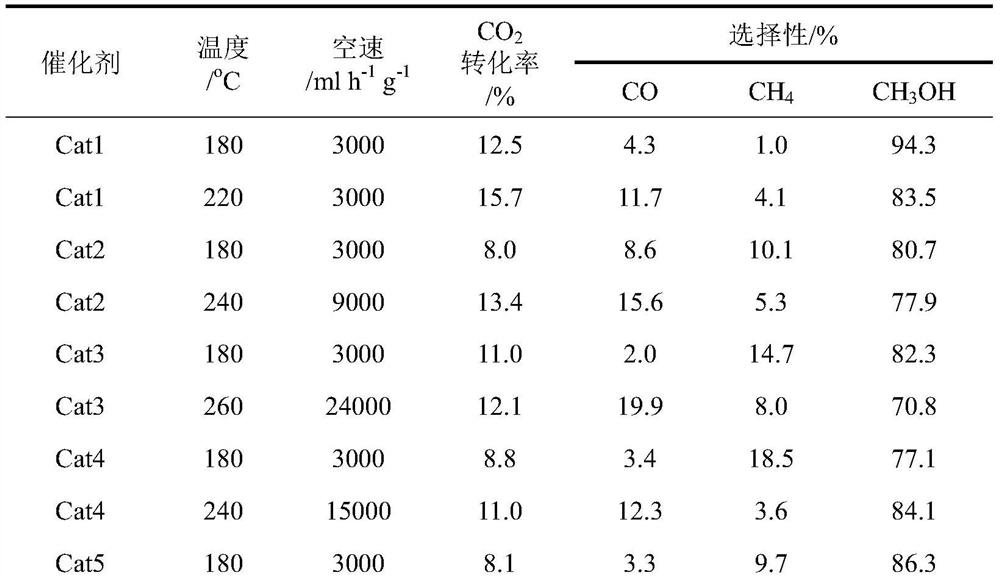
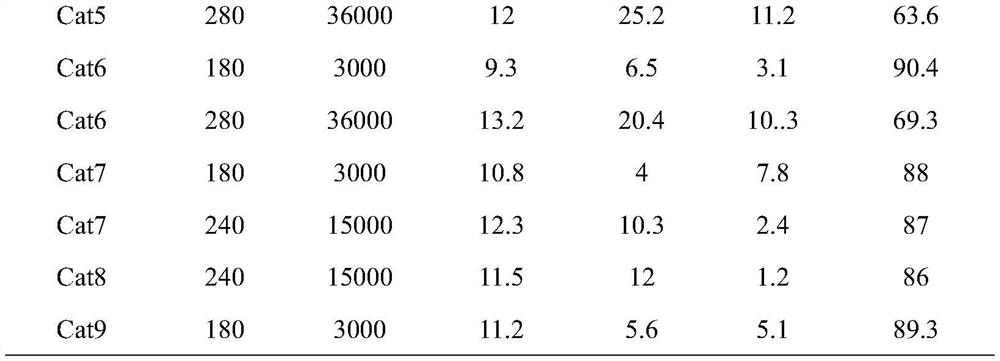
[0022] For the convenience of comparison, the evaluation conditions and results of the application of the catalyst for carbon dioxide to methanol are summarized in the application example, and the evaluation results are listed in Table 1.
Embodiment 2
[0024] 1. Seal 0.73mmol of ammonium molybdate and 10ml of carbon disulfide into a 40ml autoclave under nitrogen protection;
[0025] 2. Keep the autoclave in (1) at 400°C for 4 hours to obtain a sample solution;
[0026] 3. Put the sample solution obtained in (2) in 6 mol L -1 Treated in NaOH solution for 3 hours, then washed with water and ethanol for several times and suction filtered to obtain the sample;
[0027] 4. After drying the sample obtained in (3) at 100° C. for 12 hours, a sample of catalyst Cat2 was obtained.
[0028] For the convenience of comparison, the evaluation conditions and results of the application of the catalyst for carbon dioxide to methanol are summarized in the application example, and the evaluation results are listed in Table 1.
Embodiment 3
[0030] 1. 1mmol ammonium molybdate and 30mmol thiourea are dissolved in 35ml water, sealed in 100ml reactor under nitrogen protection;
[0031] 2. The reactor in (1) was kept at 200°C for 24 hours to obtain the sample solution;
[0032] 3. Put the obtained sample solution of (2) in 6 mol L -1 The KOH solution was treated for 3 hours, then washed with water and ethanol for several times and suction filtered to obtain the sample;
[0033] 4. After drying the sample obtained in (3) at 100° C. for 12 hours, a catalyst Cat3 sample was obtained.
[0034] For the convenience of comparison, the evaluation conditions and results of the application of the catalyst for carbon dioxide to methanol are summarized in the application example, and the evaluation results are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com