Preparation of fusion protein in deficiency of different structural domains and application of fusion protein to improvement of protein synthesis
A fusion protein and protein synthesis technology, applied in the field of genetic engineering, can solve the problems of low efficiency, slow speed, and limited application of protein synthesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0161] In the present invention, the preparation method of the cell extract is not limited, and a preferred preparation method includes the following steps:
[0162] (i) providing cells;
[0163] (ii) washing the cells to obtain washed cells;
[0164] (iii) performing cell disruption treatment on the washed cells to obtain a crude cell extract;
[0165] (iv) performing solid-liquid separation on the crude cell extract to obtain the liquid part, which is the cell extract.
[0166] In the present invention, the solid-liquid separation method is not particularly limited, and a preferred method is centrifugation.
[0167] In the present invention, the centrifugation conditions are not particularly limited, and a preferred centrifugation condition is 5000-100000×g, preferably 8000-30000×g.
[0168] In the present invention, the centrifugation time is not particularly limited, and a preferred centrifugation time is 0.5min-2h, preferably 20min-50min.
[0169] In the present inventi...
Embodiment 1
[0182] Embodiment 1 Improves the theoretical model of protein synthesis by genetic modification
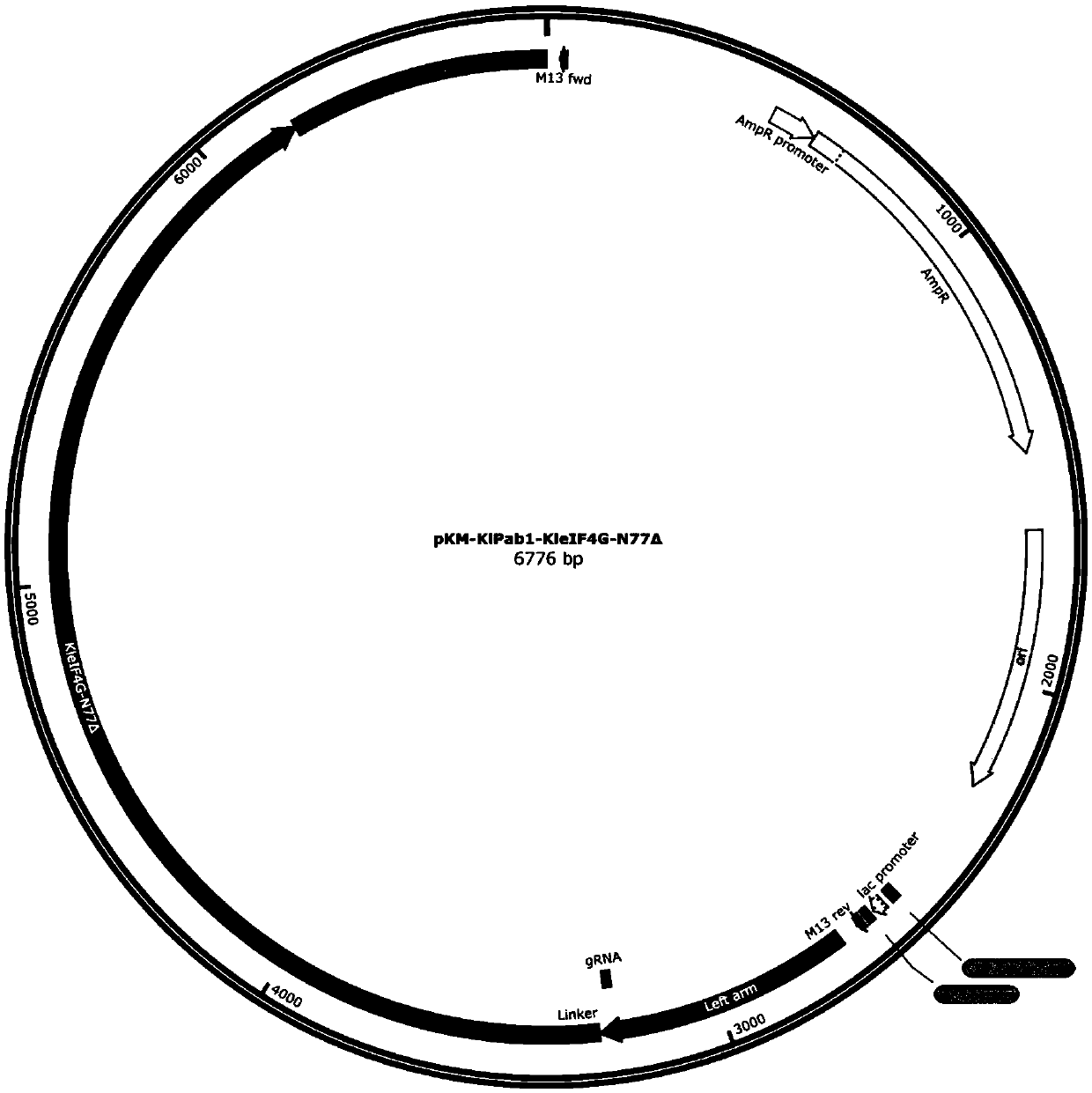
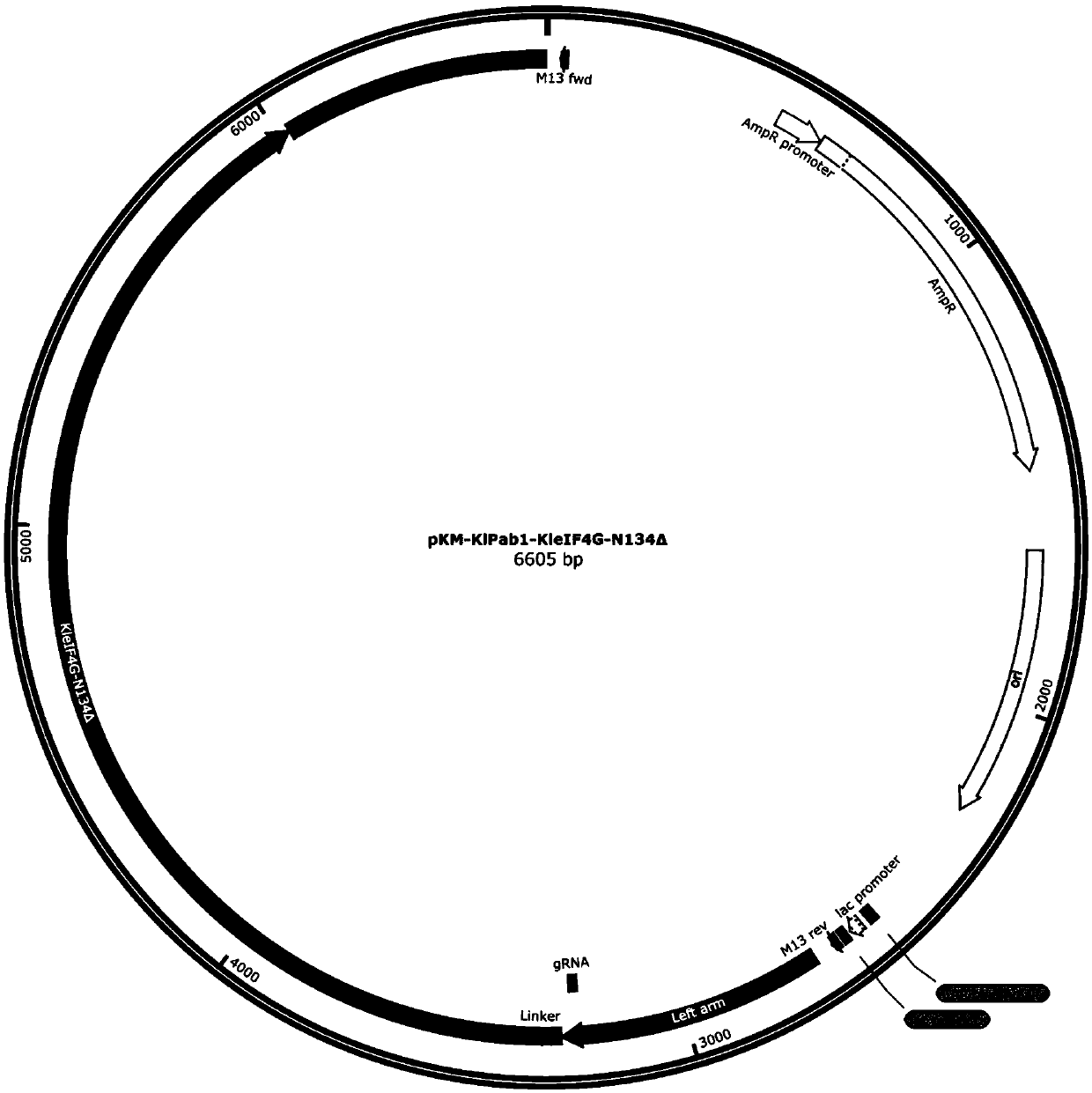
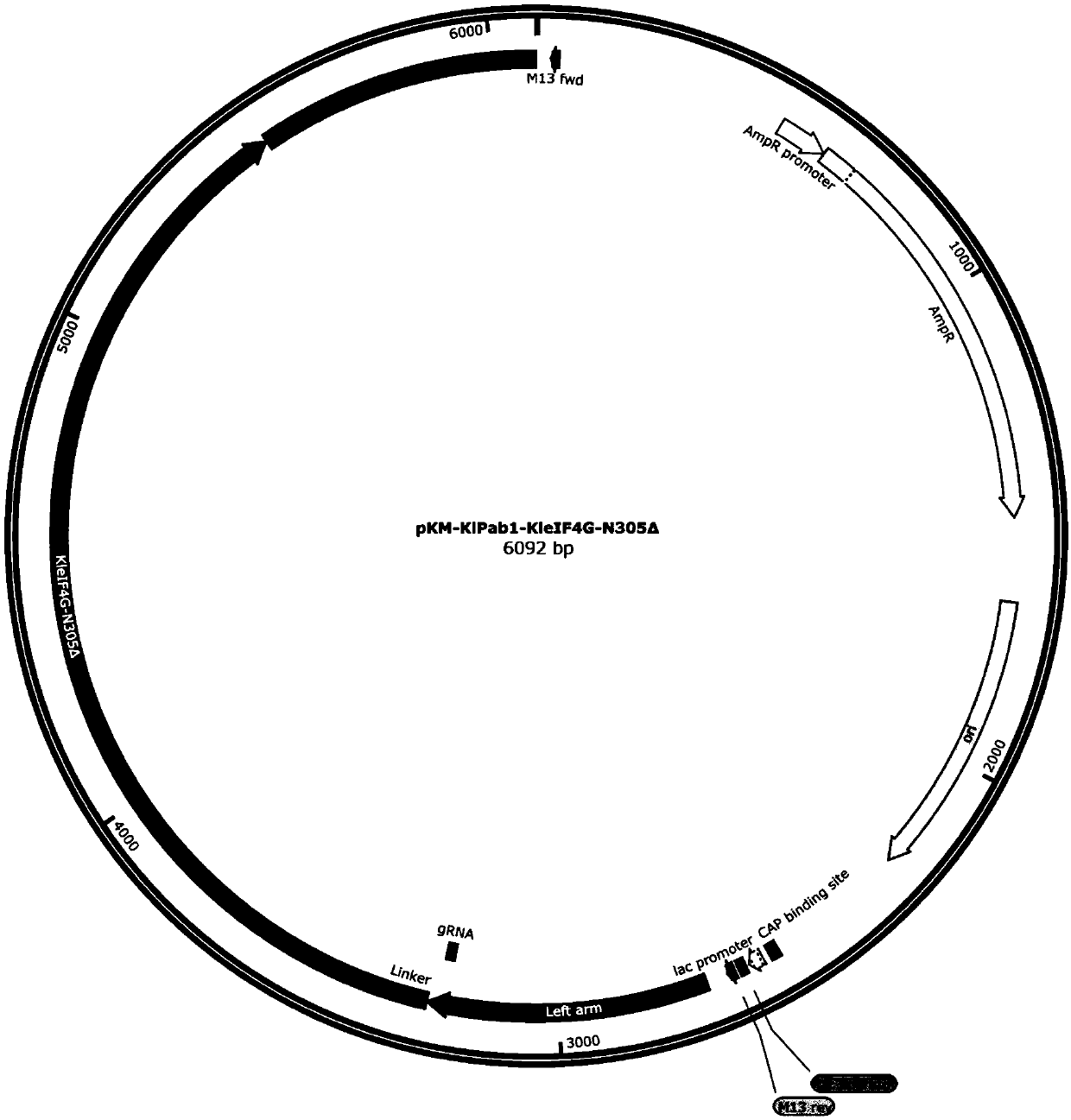
[0183] In the previous application, the inventors used CRISPR-Cas9 gene editing technology to connect a complete eIF4G protein to the C-terminus of the endogenous PAB1 (KlPAB1) protein in K. lactis, which significantly improved the efficiency of the cell-free in vitro translation system. The eIF4G protein contains multiple domains that interact with different RNA or protein elements. Some of these domains may not be involved in the process of in vitro translation, so this patent constructed a series of eIF4G (KleIF4G-N77Δ (RNA1domain deletion), KleIF4G-N134Δ (region between RNA1+RNA1 and PABP) with different domain deletions deletion), KleIF4G-N305Δ (deletion of region before PABP+PABP), KleIF4G-N566Δ (deletion of region before eIF4E+eIF4E), KleIF4G-C570Δ (deletion of region after RNA2+), KleIF4G-C605Δ (deletion of region after HEAT / eIF4A+ deletion), KleIF4G-C939Δ (RNA3domain del...
Embodiment 2
[0186] Plasmid Construction of Example 2 Deletion of Different Structure Domains
[0187] The construction of the plasmid in this example is completed on the basis of the constructed plasmid in the previous application, and the construction method of the constructed plasmid is detailed in the previous application (application number 2017106425174).
[0188] 1. Construction and amplification of KlPAB1-KleIF4G-N77Δ donor DNA plasmid
[0189] Using the pKM-KlPab1-KleIF4G-DD plasmid in the previous application as a template, PCR amplification was performed with primer PF: TTGGTGGAGGTGGATCTAACCAACCAGCGTACGGTG (SEQ ID NO.: 19) and primer PR: AGATCCACCTCCACCAACAGTAG (SEQ ID NO.: 20). Mix 17 μL of the amplification product, 1 μL of DpnI, and 2 μL of 10×digestion buffer, and incubate at 37°C for 3 hours. Add 10 μL of the DpnI-treated product to 100 μL DH5α competent cells, place on ice for 30 min, heat shock at 42 °C for 45 s, add 1 mL of LB liquid medium and shake at 37 °C for 1 h, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com