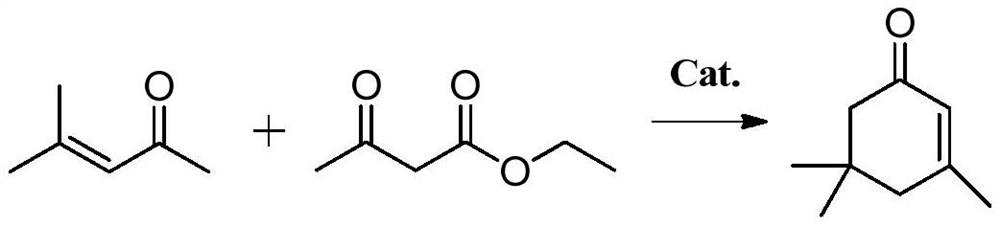
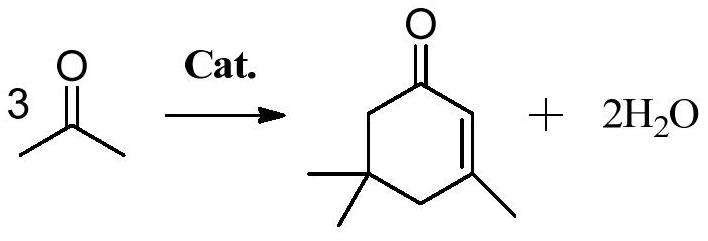
Preparation method of α-isophorone
A technology of isophorone and acetone, applied in the field of preparation of α-isophorone, can solve the problems of difficult to accurately control the residence time of reactants, low selectivity of α-isophorone, unsatisfactory acetone conversion rate and the like , to achieve the effect of low temperature and pressure, short reaction time and lower energy consumption cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The embodiment of the present invention provides a preparation method of α-isophorone, which is characterized in that it comprises the following steps:
[0038] Acetone and a hydrotalcite-based composite oxide catalyst are subjected to a polycondensation reaction under supercritical conditions, and the structural formula of the hydrotalcite-based composite oxide catalyst is Sr x Mg 0.7-x Al 0.2-y m y O, wherein, M is selected from one of Pr, Ga, and In, 0.05≤x≤0.35, 0.02≤y≤0.1;
[0039] The components in the product of the above polycondensation reaction are separated to obtain acetone multimer, and the acetone multimer and water are hydrolyzed in a high-gravity reactor.
[0040] The preparation method of α-isophorone that the embodiment of the present invention provides adopts Sr x Mg 0.7- x Al 0.2-y m y O-type hydrotalcite-based composite oxides are used as catalysts, and combined with the hydrolysis reaction steps under high gravity conditions, the mass trans...
Embodiment 1
[0063] (1) Catalyst 5gSr 0.1 Mg 0.6 Al 0.15 PR 0.05 O and 500g acetone (mass ratio is the ratio of 0.01:1) are mixed uniformly to obtain suspension, and this suspension carries out polycondensation reaction at 8MPa, 270 ℃ of supercritical conditions in tubular reactor, and the reaction time is 1min, suspending The turbid liquid was injected into the tubular reactor at a flow rate of 10 g / min. After the polycondensation reaction, the reaction liquid in the tubular reactor was cooled, and the sample was detected by GC, and the conversion rate of acetone was calculated to be 39.3%, and the selectivity of α-isophorone was 90.1%.
[0064] (2) After the polycondensation reaction finishes, each component of the reaction solution in the tubular reactor is further separated by rectification (each component separated by rectification comprises acetone, isophorone, acetone polymer (acetone four polymer and acetone pentamer), water and other impurities), and the separated acetone poly...
Embodiment 2~ Embodiment 16
[0066] Take acetone as benchmark, the quality of acetone is constant, adjust catalyst and acetone mass ratio and adjust each reaction condition (supercritical pressure, supercritical temperature, polycondensation reaction time, suspension flow rate namely flow rate) in the polycondensation reaction under acetone supercritical condition ), repeat embodiment 1, and reaction result is as shown in table 1. In addition, adjust the mass ratio of acetone multimer and water and each reaction condition (hydrolysis reaction temperature, hydrolysis reaction time, supergravity reactor stirring speed is stirring speed) in the mass ratio of acetone multimer and the high gravity hydrolysis reaction of acetone multimer, repeat embodiment 1, The results are shown in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com