Preparation method of carbetocin injection
A technology of carbetocin and injection, which is applied in the field of preparation of carbetocin injection, can solve the problems of large increase of impurities and unresolved problems, and achieve the effect of good and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
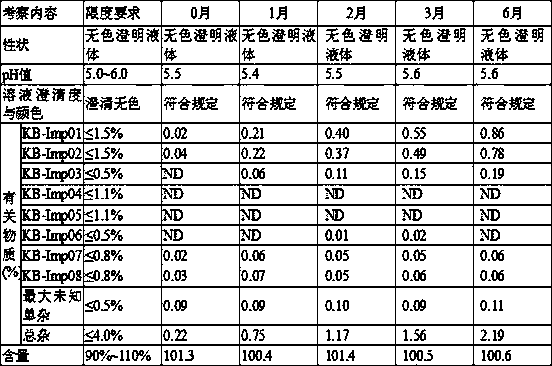
[0031] Preparation of Carbetocin Injection
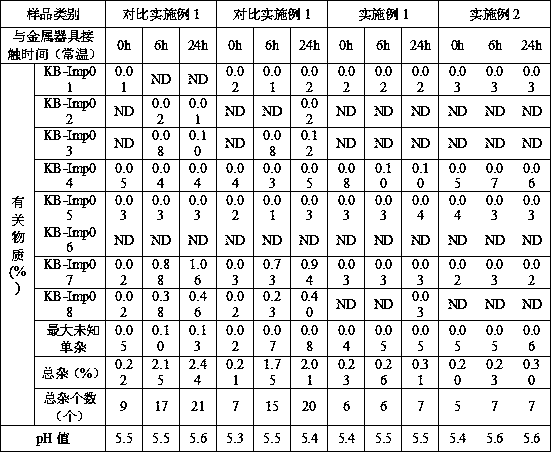
[0032] 1) Metal utensils are first soaked in an aqueous solution containing 0.1% edetate disodium, heated to 40°C and kept warm for 1 hour, then rinsed with water for injection, and sterilized for later use;
[0033] 2) Dissolve carbetocin and excipients, add water to volume, and stir to mix completely;
[0034] 3) 0.22μm sterile filtration;
[0035] 4) Filling and capping, ready to serve.
Embodiment 2
[0037] Preparation of Carbetocin Injection
[0038] 1) Metal utensils are soaked in an aqueous solution containing 0.05% calcium sodium edetate, heated to 60°C and kept warm for 2 hours, then rinsed with water for injection, and sterilized for later use;
[0039] 2) Dissolve carbetocin and excipients, add water to volume, and stir to mix completely;
[0040] 3) 0.22μm sterile filtration;
[0041] 4) Filling and capping, ready to serve.
Embodiment 3
[0043] Preparation of Carbetocin Injection
[0044] 1) Metal utensils are soaked in an aqueous solution containing 0.5% calcium sodium edetate, heated to 50°C and kept warm for 1 hour, then rinsed with water for injection, and sterilized for later use;
[0045] 2) Dissolve carbetocin and excipients, add water to volume, and stir to mix completely;
[0046] 3) 0.22μm sterile filtration;
[0047] 4) Filling and capping, ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com