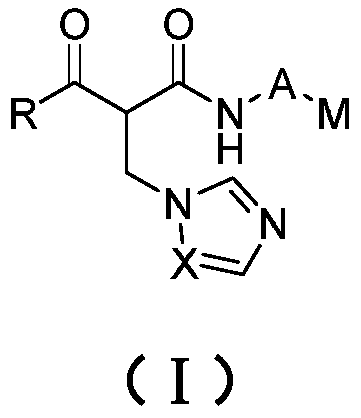
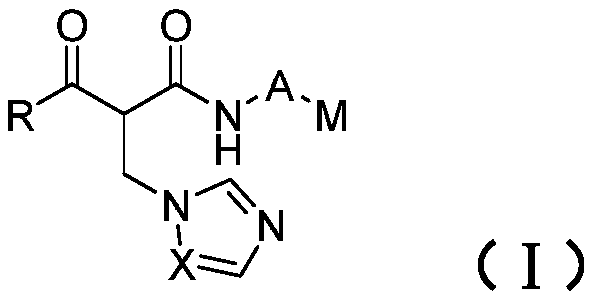
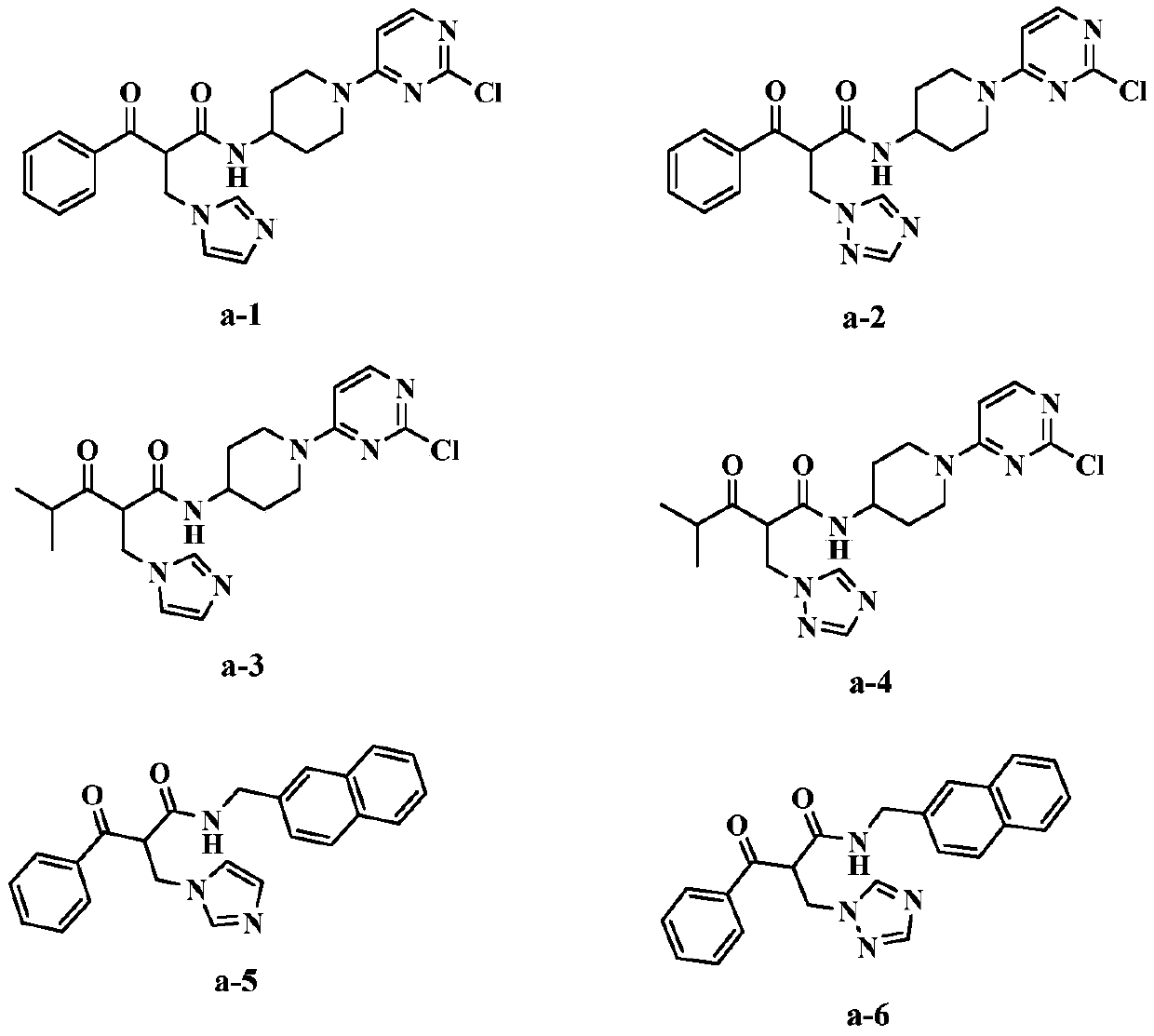
Formyl acetamide azole derivative and use thereof
A technology of azole derivatives and formylacetamide, applied in the field of drug synthesis, can solve the problems of low recurrence rate, high drug resistance and metabolic toxicity, and difficult to overcome.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 2-((1H-imidazol-1-yl)methyl)-N-(1-(2-chloropyrimidin-4-yl)piperidin-4-yl)-3-oxo-3-benzene Preparation of propionamide
[0054] Step 1 Preparation of (1-(2-chloropyrimidin-4-yl) piperidin-4-yl) tert-butyl carbamate
[0055] Dissolve 2,4-dichloropyrimidine (1.0eq) in DMF solution, add K 2 CO 3 (1.5eq), under stirring, N-(piperidin 4-yl) tert-butyl carbamate (1.2eq) was added, and reacted at room temperature for 6 hours. After the reaction was complete, ice water was added, and a solid was precipitated, which was filtered and dried to obtain the obtained compound is required.
[0056] Step 2 Preparation of 1-(2-chloropyrimidin-4-yl)piperidin-4-amine
[0057] Dissolve tert-butyl (1-(2-chloropyrimidin-4-yl)piperidin-4-yl)carbamate (1.0eq) in dichloromethane solution, add excess trifluoroacetic acid (10.0 eq), adding saturated K 2 CO 3 The solution was adjusted to alkali, extracted with dichloromethane, and the organic layer was collected Na 2 SO 4 , dry ov...
Embodiment 2
[0065] Example 2 2-((1H-1,2,4-triazol-1-yl)methyl)-N-(1-(2-chloropyrimidin-4-yl)piperidin-4-yl)-3 - Oxo-3-phenylpropanamide;
[0066] Yield: 72.3%; mp: 145.9–148.2℃. 1 H NMR (400MHz, DMCO-d 6 )δ8.77(s,1H),8.39–7.93(m,2H),7.84(dd,J=40.6,9.2Hz,1H),7.67(ddt,J=16.5,13.6,3.1Hz,1H),7.56 –7.28(m,1H),6.70(s,1H),6.16(d,J=15.0Hz,1H),5.23(dd,J=24.7,8.6Hz,1H),4.92–4.73(m,1H), 4.37(dd,J=24.7,8.6Hz,1H),3.83(p,J=15.2Hz,1H),3.53–3.00(m,2H),2.12–1.86(m,1H),1.74(ddt,J= 24.8,15.2,11.0Hz,1H). 13 C NMR (101MHz, DMSO-d 6 )δ196.32,168.60,163.89,156.72,156.18,151.75,144.06,137.04,132.74,129.14,128.61,97.18,54.13,52.79,48.55,47.50,30.70.ESI-MS m / z:44 + ;462.2[M+Na] + ;4387.2[M-H] - .
Embodiment 3
[0067] Example 3 2-(((1H-imidazol-1-yl)methyl)-N-(1-(2-chloropyrimidin-4-yl)piperidin-4-yl)-4-methyl-3- Oxyvaleramide;
[0068] Yield: 68.7%; mp: 143.3–146.7℃. 1 H NMR (400MHz, DMCO-d 6)δ7.92(s,1H),7.80(d,J=15.0Hz,1H),7.18(d,J=15.0Hz,1H),6.78(dd,J=15.0,0.6Hz,1H),6.35( s,1H),6.16(d,J=15.0Hz,1H),4.39(dd,J=23.3,12.8Hz,1H),4.26(dd,J=14.6,11.0Hz,1H),4.14(dd,J =23.2,12.7Hz,1H),3.72(p,J=15.2Hz,1H),3.49–3.13(m,4H),2.69–2.33(m,1H),2.04–1.80(m,2H),1.65( ddt,J=24.8,15.2,11.0Hz,2H),1.04(d,J=12.8Hz,6H). 13 C NMR (101MHz, DMSO-d 6 )δ212.74,167.16,163.89,156.72,156.18,139.84,128.74,120.63,97.18,61.33,49.12,48.55,47.50,38.24,30.70,18.56.ESI-MS m / z:405.2[M+H] + ;427.2[M+Na] + ;403.2[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com