Method for introducing CRISPR-Cas9 system into human stem cells
A stem cell, hbb-sgRNA-cas9-t2a-gfp-sz technology, applied in the field of gene editing, can solve problems such as cell damage, and achieve the effects of fast expression time, high safety, and improved cell transfection efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The introduction of the CRISPR-Cas9 gene editing system into human umbilical cord-derived mesenchymal stem cells (human umbilicalcord-derived mesenchymal stem cells, hMSC) is used as a specific example to describe the patent of the invention in detail.
[0048] 1. Construction of HBB-sgRNA-Cas9-T2A-GFP-SZ plasmid carrying gene editing
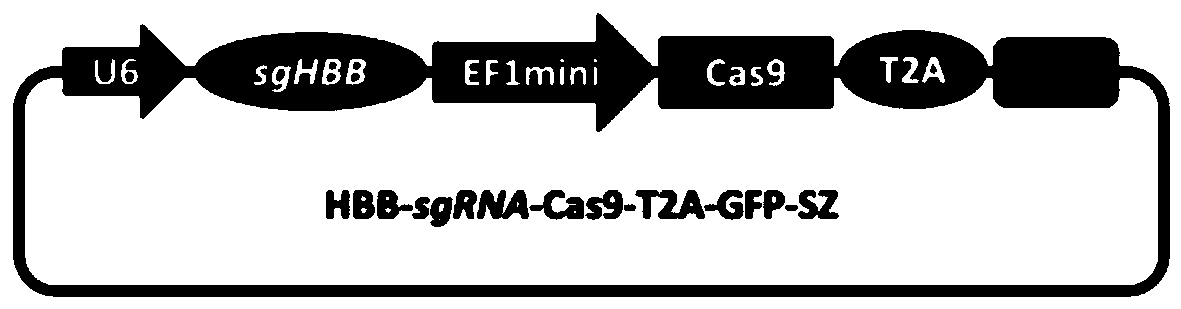
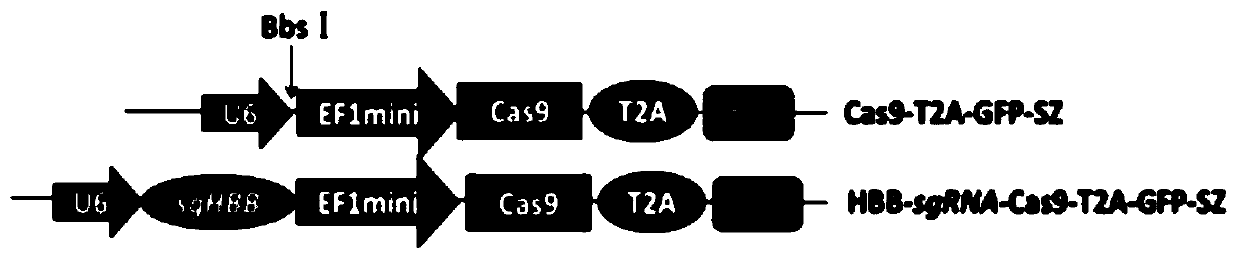
[0049] 1.1 Transform the EF1 promoter to drive Cas9 to reduce the size of the imported gene fragment, chemically synthesize the CRISPR-Cas9 expression cassette and connect the GFP expression gene to construct the Cas9-T2A-GFP-SZ backbone plasmid; at the same time, target the human hemoglobin gene in hemophilia B (HBB, human haemoglobin beta), designed a highly targeted HBB-sgRNA, connected with the Cas-T2A-GFP-SZ plasmid to form a recombinant plasmid HBB-sgRNA-Cas9-T2A-GFP-SZ ( Figure 1-2 ).
[0050] The following is part of the DNA sequence of the HBB-sgRNA carrier (the gRNA sequence containing the HBB gene, that is, the underlined se...
Embodiment 2
[0114] The invention patent is described in detail by introducing the CRISPR-Cas9 gene editing system into human umbilical cord blood-derived hematopoietic stem cells (UB-HSC, umbilical cord blood-derived hematopoietic stem cells) as a specific example.
[0115] There are many sources of hematopoietic stem cells, such as bone marrow and peripheral blood, but compared with adult-derived hematopoietic stem cells, UB-HSC contains more early hematopoietic stem cells and has higher differentiation potential than adult-derived hematopoietic stem cells. At present, hematopoietic stem cell transplantation is used to treat various diseases, and the combination of UB-HSC and CRIPSR-Cas9 editing system has a good application prospect. However, UB-HSC is a kind of suspended stem cell, and it is a major difficulty in this technology to introduce this system efficiently into UB-HSC. The invention of this patent can make full use of the advantages of electroporation transfection to introduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com